Abstract
Achievement of malaria elimination requires development of novel strategies interfering with parasite transmission, including targeting the parasite sexual stages (gametocytes). The formation of Plasmodium falciparum gametocytes in the human host takes several days during which immature gametocyte-infected erythrocytes (GIEs) sequester in host tissues. Only mature stage GIEs circulate in the peripheral blood, available to uptake by the Anopheles vector. Mechanisms underlying GIE sequestration and release in circulation are virtually unknown. We show here that mature GIEs are more deformable than immature stages using ektacytometry and microsphiltration methods, and that a switch in cellular deformability in the transition from immature to mature gametocytes is accompanied by the deassociation of parasite-derived STEVOR proteins from the infected erythrocyte membrane. We hypothesize that mechanical retention contributes to sequestration of immature GIEs and that regained deformability of mature gametocytes is associated with their release in the bloodstream and ability to circulate. These processes are proposed to play a key role in P falciparum gametocyte development in the host and to represent novel and unconventional targets for interfering with parasite transmission.
Introduction
An essential step in the achievement of malaria elimination is to block the transmission of sexual stages parasites, the gametocytes, to the mosquito vector. In the case of Plasmodium falciparum, causing the most lethal form of malaria, gametocyte maturation requires the exceptionally long time of 8 to 10 days, compared with the 48-hour asexual cycle, and is conventionally divided into 5 different morphologic stages (I-V).1 Only the mature stage V circulate in the peripheral blood, whereas immature gametocyte-infected erythrocytes (GIEs) from stage II to stage IV have been reported to sequester in internal organs, such as the bone marrow and the spleen.2,3 Although profound morphologic changes, accompanied by expression of 2 to 300 sexual stage–specific transcripts and proteins, have been described in gametocytogenesis,4-6 the mechanisms of GIE sequestration and the relative contribution of cytoadherence and changes in GIE deformability in this process are virtually unknown.
Proposed mechanisms of P falciparum sequestration mainly derive from studies on the pathogenic asexual stages. These circulate in the bloodstream as “ring” stages in the first 24 hours after erythrocyte invasion, and then sequester in various organs to complete maturation to schizont stages, which burst to produce the next generation of free circulating ring forms. Asexual parasite sequestration is mediated by parasite-induced modifications of the erythrocyte surface called knobs, enabling the interaction of P falciparum erythrocyte membrane protein 1 (PfEMP1) with host ligands on microvasculature endothelial cells. The absence of knobs in gametocytes from stage II to stage V and their failure to adhere to endothelial cell lines7 as well as failure to detect PfEMP1 on the surface of erythrocytes infected by stage III and stage IV gametocytes8-11 suggest, however, that maintenance of sequestration of immature GIEs is mediated by different mechanisms. Other families of genes involved in host cell modification, such as RIFINs and STEVORs, are expressed during gametocytogenesis,12,13 but a functional role for such proteins in sexual differentiation has not yet been demonstrated. STEVOR proteins, produced by transcripts expressed early in gametocytogenesis, are trafficked to the infected erythrocyte membrane during gametocyte maturation.12 STEVORs have been recently shown to strongly impact deformability of erythrocytes hosting asexual P falciparum parasites.14
In this work, we analyzed the rheologic properties of GIEs at various stages of development, complementing such observations with a molecular and cellular analysis of STEVOR expression and localization during gametocytogenesis. Ektacytometry and microsphiltration methods were combined here, for the first time, to measure GIE deformability and filterability, respectively, of P falciparum gametocytes. Such technically diverse approaches indicated that immature GIEs are poorly deformable and revealed that mature stage V GIEs are significantly more deformable than immature GIEs. Moreover, we show that STEVOR proteins contribute to the overall stiffness of immature GIEs and that the observed switch in cellular deformability is linked to the deassociation of STEVORs from the erythrocyte membrane in mature gametocytes.
Methods
Gametocyte culture and stage-specific purification
The P falciparum clonal lines 3D7, B10, H4, and A12 as well as the transgenic lines SFM (Stevor-Flag-c-Myc), 2TMFM (Pfmc-2TM-FLAG-myc), and 3D7GFP have been described elsewhere.4,15,16 All derive from the NF54 line. Parasites were cultivated in vitro under standard conditions using RPMI 1640 medium supplemented with 10% heat-inactivated human serum and human erythrocytes at a 5% hematocrit.17 Synchronous production of gametocytes stages was achieved as described.18 For the isolation of gametocytes, culture medium was supplemented with 50mM N-acetylglucosamine from day 0 onwards, and medium replacement was continued for 2 to 5 days to eliminate the asexual stages. Gametocytes were enriched by Percoll gradient or by magnetic isolation using a MACS depletion column (Miltenyi Biotec) in conjunction with a magnetic separator.
Immature GIEs from patient blood
A 42-year-old patient was treated with quinine for severe malaria attack with impaired consciousness, renal failure, and 4% initial parasitemia composing a large proportion of mature trophozoites and schizonts and a smaller proportion of immature gametocytes. Howell-Jolly bodies, a marker of hyposplenism, were evident in 0.3% of red blood cells, thereby explaining the very unusual aspect of the thin smear. Immunochromatography and PCR confirmed that the patient was infected exclusively with P falciparum. At day 3 and day 4 of quinine therapy, blood was collected to assess parasite clearance and sent the same day to the National Reference Center for Malaria. Thin smears showed complete clearance of asexual stages and the persistence of immature GIEs (stages II-IV). Blood samples were washed 3 times with RPMI medium to remove leucocytes and immediately submitted to microsfiltration as described. GIEs were staged and enumerated on 200 high-power (×1000) fields and the retention rate calculated as described. Patient consent was obtained by the attending physician before blood collection as per National Reference Center standard operating procedure.
Ektacytometry measurement of GIE population EI
Deformability measurements of GIE populations were carried out using ektacytometry analysis via laser-assisted optical rotational cell analyzer (LORCA).19 The extent of erythrocyte deformability, or elongation index (EI), was defined as the ratio between the difference of the 2 axes of the ellipsoid diffraction pattern and the sum of these 2 axes. Populations of GIE stage II/III and stage V, at 40% parasitemia and 40% hematocrit in a final volume of 25 μL, were diluted in isotonic solutions of polyvinylpyrrolidone and exposed to increasing shear stresses from 0 to 30 Pa, at 37°C, as described.19 Gametocytes were enriched by multilayer Percoll gradient from synchronized cultures. At least 2 independent experiments were performed for each GIE stage. Each independent experiment included a Percoll-treated population of uninfected erythrocytes from the same batch and kept in culture for the same time of the GIE, at 40% hematocrit in a final volume of 25 μL. Direct microscopic observation of GIEs diluted in polyvinylpyrrolidone was performed before and after LORCA measurements and excluded that cell aggregation was occurring in the experiments.
Microsphiltration
Calibrated metal microspheres (96.50% tin, 3.00% silver, and 0.50% copper; Industrie des Poudres Sphériques) with 2 different size distributions (5- to 15-μm-diameter and 15- to 25-μm-diameter) composed a matrix used to assay infected erythrocyte deformability under flow, as recently described.20,21 Suspensions of synchronized cultures containing 2% to 5% GIEs were perfused through the microsphere matrix at a flow rate of 60 mL/hour using an electric pump (Syramed μsp6000, Arcomed′ Ag), followed by a wash with 6 mL complete medium. The upstream and downstream samples were collected and smeared onto glass slides for staining with Giemsa reagent, and parasitaemia was assayed to determine parasite retention versus flow-through. To visualize GIE shape during their flowing through the matrix, 1 mL of PBS/4% paraformaldehyde was added after perfusion of the GIE-containing culture on the microsphere matrix. After 5 minutes of incubation, fixed GIEs were separated from the microspheres by a 3-step decantation procedure, and GIE morphology was observed on a glass slide by light microscopy using a Leica DM 5000 B at 100× magnification.
Scanning electron microscopy
Before gametocytogenesis induction, the P falciparum B10 clone was selected by gel floatation during several cycles to select for knob-producing parasites. Gametocytes were purified by magnetic isolation and the cell pellets resuspended in 2.5% gluteraldehyde (EM grade) in sodium cacodylate 0.1M, pH 7.2, for 1 hour at 4°C. Cells were washed 3 times in sodium cacodylate, transferred to polylysine-coated coverslips, and incubated 1 hour in 1% osmium tetroxide. After 3 washes in H2O, samples were dehydrated (25%, 50%, 75%, 95%, 2 × 100%, 5 minutes each), incubated for 10 minutes in acetone, subjected to critical point drying, and coated with platinum in a gun ionic evaporator. Samples were examined and photographed with a JEOL 6700 F electron microscope operating at 2 kV.
Immunostaining of fixed and live GIEs
Parasites were washed in PBS, air-dried on glass blood smears, and methanol- or acetone-fixed for 5 to 15 minutes. After 1-hour preincubation in 1% BSA, parasites were incubated with one of the following antisera: anti–STEVOR mouse antiserum (mouse anti-S2) diluted 1:400,22 a pool of mouse antisera against 4 STEVOR proteins (anti-PFA0750w, -PFL2610w, -MAL13P1.7, or -PFC0025c) diluted 1:500,23 an anti-Pfg27 rabbit antiserum diluted 1:100,24 or an anti–c-myc rat monoclonal antibody diluted 1:500 (Santa Cruz Biotechnology). After washes in PBS, slides were incubated with AlexaFluor-conjugated secondary antibody against rat, rabbit, or mouse IgGs (Invitrogen) containing 4,6-diamidino-2-phenylindole (DAPI; 2 μg/mL). Samples were observed at 100× magnification using a Leica DM 5000 B or an Olympus fluorescent microscope. Immunostaining of live GIEs was performed on MACS-purified GIEs washed in RPMI, resuspended in binding buffer (RPMI/10% FBS), and diluted to 5% parasitemia with uninfected erythrocytes. A total of 50 μL of cell suspension was incubated for 1 hour at 4°C in a rotating wheel with rabbit anti-STEVOR (rabbit anti-S2)25 and mouse monoclonal anti–Glycophorin C (GPC; vCell Science) diluted 1:400 and 1:500, respectively. For detection of Pfg27 and/or STEVOR internal proteins, infected erythrocytes were permeabilized by addition of 3 to 4 hemolytic units of streptolysin O (Sigma-Aldrich) as described.26 After 3 washes in RPMI, parasites were incubated 1 hour with AlexaFluor-conjugated secondary antibody against either rabbit or mouse IgGs (Invitrogen) containing DAPI (2 μg/mL). Samples were mounted in vectashield onto a glass slide and visualized with Olympus fluorescence microscope at 100× magnification.
Immunoblotting analysis
GIEs were purified by magnetic isolation and denatured in protein loading buffer 5 minutes at 100°C. Samples (5 × 106 parasites/lane) were separated by 4% to 12% SDS-PAGE, transferred to PVDF membrane, and blocked for 1 hour in 5% nonfat dry milk. Immunoblots were probed with a pool of mouse antiserum against STEVOR proteins (anti-PFA0750w, -PFL2610w, -MAL13P1.7, or -PFC0025c) at 1:3000,23 a mouse mAb anti-HSP70 antibody at 1/5000, followed by 1 hour with HRP-conjugated anti–mouse IgG secondary antibodies (Promega) at 1:25 000. Detection step was performed using the Pierce chemoluminescence system (Pierce Chemical) following the manufacturer's instructions.
Statistical analysis
Statistical significance for differences in EIs and retention rates were established using Wilcoxon Mann-Whitney rank-sum test. Statistical significance for differences in proportion of GIE showing different shape was established using the χ2 test.
Results
Mature GIEs are more deformable than immature GIEs
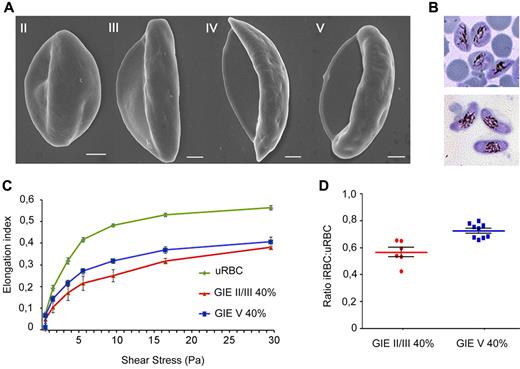
Scanning electron microscopy of the conventionally described stages of gametocyte maturation1,5 showed the evolution from a convex half moon-like shape in stage II to an elongated shape in stage III, followed by a crescent shape with protruding extremities in stage IV, finally leading to an elongated and curved shape with smooth ends in the mature stage V (Figure 1A). Interestingly, the process is accompanied by an increasing transparency of the residual portion of erythrocyte cytoplasm (Laveran bib), consistent with the reported decrease in hemoglobin concentration in developing gametocytes.27 To investigate whether these morphologic changes are associated with changes in mechanical properties of infected cells, we used ektacytometry via LORCA. In these experiments, the EI is measured in response to increasing shear stress (SS) from 0 to 30 Pa, with higher EI corresponding to increased GIE deformability. Cell samples containing 40% infected erythrocytes with either immature (stage II/III) or mature (stage V) 3D7 gametocyte stages were compared (Figure 1B). A result of these experiments was that mature GIE populations consistently showed higher EI values over all SS compared with the immature GIE samples in independent biologic replicates (Figure 1C). To eliminate interference of factors, such as blood source or culture time, the average ratio for EI of infected versus uninfected erythrocytes was calculated for each SS. In the SS range from 3 to 9.49 Pa, more sensitive to detect differences in deformability between erythrocyte populations compared with higher SS,28 ratio was significantly lower (P = .0004) for stage II/III GIEs compared with the mature stage V GIEs (Figure 1D). Significantly different ratios persisted at higher SS (supplemental Figure 1; see the Supplemental Materials link at the top of the article), although both cell types tended to reach closer EI values at maximum SS of 30 Pa, a phenomenon previously observed in studies on blood diseases where erythrocytes present decreased deformability.28 In summary, these results clearly indicate that mature GIEs are significantly more deformable than erythrocytes containing the immature II/III sexual stages.
Scanning electron microscopy and ektacytometry analysis of immature and mature P falciparum GIEs. (A) Scanning electron microscopy images of Plasmodium falciparum GIEs (B10 clone) from stage II to stage V of maturation. Bars represent 1 μm. (B) Giemsa staining images of stage II/III (top panel) and stage V (bottom panel) GIE samples used in ektacytometry analysis. (C) Response to increasing shear stress of erythrocytes infected by P falciparum stage II/III (red line) and stage V (blue line) gametocytes (40% parasitemia) and of uninfected erythrocytes (green line). Error bars represent SE. (D) Ratios of EIs of infected versus uninfected erythrocytes calculated from the 3- to 9.49-Pa range of shear stresses in the ektacytometry analysis (C) showing a statistically significant difference between immature and mature GIEs (Mann-Whitney rank-sum test, P = .0004).
Scanning electron microscopy and ektacytometry analysis of immature and mature P falciparum GIEs. (A) Scanning electron microscopy images of Plasmodium falciparum GIEs (B10 clone) from stage II to stage V of maturation. Bars represent 1 μm. (B) Giemsa staining images of stage II/III (top panel) and stage V (bottom panel) GIE samples used in ektacytometry analysis. (C) Response to increasing shear stress of erythrocytes infected by P falciparum stage II/III (red line) and stage V (blue line) gametocytes (40% parasitemia) and of uninfected erythrocytes (green line). Error bars represent SE. (D) Ratios of EIs of infected versus uninfected erythrocytes calculated from the 3- to 9.49-Pa range of shear stresses in the ektacytometry analysis (C) showing a statistically significant difference between immature and mature GIEs (Mann-Whitney rank-sum test, P = .0004).
A switch in deformability and filterability of GIEs occurs at the transition between immature and mature gametocytes
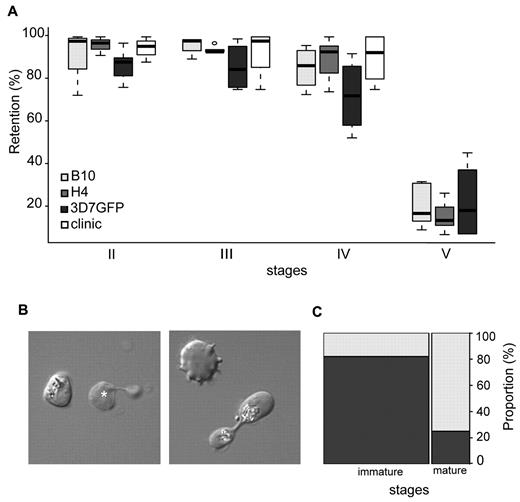
To evaluate GIE mechanical properties in an independent and technically diverse approach, the filterability of GIEs using the microsphiltration method was analyzed. This technique measures deformability of parasitized erythrocyte flowing through a defined matrix of microspheres that contains narrow and short apertures, and was validated against ex vivo perfused human spleen.20,21 As this method mimics the physical constraints experienced by infected erythrocytes in the splenic microcirculation, it was used to investigate whether changes in GIE deformability during gametocytogenesis correlated with the ability of different gametocyte stages to circulate in peripheral blood. In this system, increased retention rates and impaired filterability correspond to decreased erythrocyte deformability.29 Retention rates were monitored for synchronous cultures of stage II, III, IV, and V gametocytes from 3 different parasite clones (Figure 2A). In all parasite lines, immature GIEs (stages II-IV) displayed high retention rates ranging from 72% to 96%. Experiments with immature GIEs (stages II-IV) from the blood of a hyposplenic patient confirmed this high retention rate (75%-100% retention), indicating that this property is not the result of an in vitro artifact linked to gametocyte cultivation. In marked contrast, mature stages were substantially more deformable because less than 23% of stage V GIEs were retained on the microspheres (P < .00001). Analysis of Giemsa-stained smears of mature gametocytes upstream and downstream the microsphere matrix showed a similar male/female ratio for the 2 samples. To visualize the shape of GIE as they flow through the matrix, we added a paraformaldehyde-fixation step in the microsphiltration experiment. This showed that 82% of observed immature stages maintained their convex shape, whereas 75% of mature gametocytes displayed a twisted, elongated dumbbell-like shape probably reflecting their ability to squeeze and slide between microspheres (Figure 2B-C). These results clearly confirm the observations obtained by ektacytometry that mature GIEs are significantly more deformable than immature GIEs. Importantly, microsphiltration experiments in addition reveal that a major switch in GIE deformability occurs at the transition between stage IV and stage V, which coincides with the release from the sequestration sites and the restored ability to circulate of the mature stages.
Retention in microsphilters of immature and mature GIEs. (A) Retention in microsphilters of stages II, III, IV, and V GIE from different P falciparum clonal lines (B10, H4, and 3D7GFP) in culture or directly collected from the blood of a hyposplenic patient treated for a malaria attack (clinic). Immature GIEs (stages II-IV) are retained by the microsphilters while mature GIEs (stage V) flow through. (B) Differential interference contrast images of paraformaldehyde-fixed GIEs as they flow through the microsphilters. Immature stages (left panel) keep a convex oval or round shape, unlike uninfected erythrocytes (white star), whereas a majority of mature GIEs are twisted and dumbbell-shaped (right panel). (C) Graphical representation for the proportion of GIEs showing a regular (dark gray) or twisted (light gray) shape in a population of immature (n = 100) and mature (n = 36) GIEs (χ2 test, P = 1 × 10−9).
Retention in microsphilters of immature and mature GIEs. (A) Retention in microsphilters of stages II, III, IV, and V GIE from different P falciparum clonal lines (B10, H4, and 3D7GFP) in culture or directly collected from the blood of a hyposplenic patient treated for a malaria attack (clinic). Immature GIEs (stages II-IV) are retained by the microsphilters while mature GIEs (stage V) flow through. (B) Differential interference contrast images of paraformaldehyde-fixed GIEs as they flow through the microsphilters. Immature stages (left panel) keep a convex oval or round shape, unlike uninfected erythrocytes (white star), whereas a majority of mature GIEs are twisted and dumbbell-shaped (right panel). (C) Graphical representation for the proportion of GIEs showing a regular (dark gray) or twisted (light gray) shape in a population of immature (n = 100) and mature (n = 36) GIEs (χ2 test, P = 1 × 10−9).
The switch in deformability correlates with a modified STEVOR localization in the erythrocyte
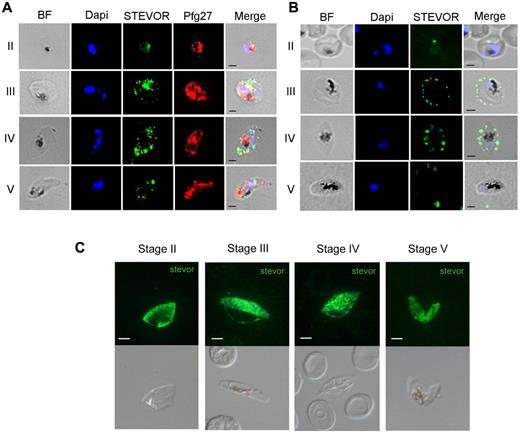
To investigate whether GIE deformability modifications are mediated by changes in expression or location of specific parasite proteins, we focused on parasite gene products known to be expressed during gametocytogenesis and associated with the erythrocyte membrane. Among them, the members of the STEVOR multigenic family fulfilled these criteria12,13,15,25 and, importantly, STEVORs were recently shown in asexual stages to impact deformability of the infected erythrocytes.14 Expression and cellular localization of STEVORs during gametocyte development were investigated by Western blot and by immunofluorescence using different sets of antibodies. Western blot analysis using mouse polyclonal sera against a semiconserved region of STEVOR proteins25 showed that STEVOR abundance was stable from stage II to stage V GIE (supplemental Figure 2). Immunofluorescence analysis of acetone-fixed GIEs using the same antibodies confirmed the presence of STEVOR from stage II to stage V GIEs (Figure 3A). STEVOR-specific fluorescence was progressively exported to the erythrocyte cytoplasm of stage III and stage IV GIEs, in which a dotted pattern colocalizing with the erythrocyte membrane was clearly visible. In stage V gametocytes, the STEVOR signal was mainly associated with the gametocyte cytoplasm, colocalizing with the signal from the gametocyte internal protein Pfg27 (Figure 3A).30 Such a differential distribution of STEVORs in immature and mature GIE was confirmed on methanol-fixed GIEs using a pool of mouse antisera against 4 STEVOR proteins (Figure 3C).23 To gain further insights in STEVOR localization, immunostaining of live GIEs showed that STEVORs could be detected on the surface of stage III and stage IV but not of stage V GIEs (Figure 3B). This observation was confirmed as anti-STEVOR antibodies gave a double-positive signal with anti–Glycophorin C antibodies only on the surface of immature GIEs and not on that of stage V GIEs (supplemental Figure 3). Furthermore, stage V gametocytes positively reacted with anti-STEVOR antibodies only after streptolysin O permeabilization, confirming that the residual STEVOR-specific signal on mature GIEs was the result of internally located STEVOR proteins (supplemental Figure 2). In summary, our data show that the switch in cellular deformability at the transition between immature and mature GIEs coincides with a moment in which STEVORs are no longer detectable in association with the erythrocyte membrane. This result suggests that STEVORs mediate a decreased erythrocyte membrane viscoelasticity and contribute to infected cell stiffness in immature GIEs, similarly to that observed for erythrocytes infected with asexual stages.14
Immunofluorescence analysis of STEVOR protein expression in GIEs. (A) Analysis of STEVOR protein expression in fixed stages II to V GIE preparations. GIEs were acetone-fixed and costained with mouse anti-S2 (green) and rabbit anti-Pfg27 (red) followed by anti–mouse Alexa-488– and anti–rabbit Alexa-594–conjugated IgG. Parasite nuclei were stained with DAPI (blue). Bright-field (BF) and merge images are shown. Bars represent 2 μm. (B) Analysis of live GIEs immunostained with rabbit anti-S2 (green) specifically recognizing native STEVOR on the surface of stages III and IV GIEs. Bound antibody was detected with anti–rabbit Alexa-488–conjugated IgG. BF, nuclear staining (DAPI, blue), and merge images are shown. (C) Immunofluorescence analysis of STEVOR expression in fixed stages II to V-GIEs. GIEs were methanol-fixed and stained with a pool of mouse polyclonal antibodies directed against STEVOR proteins followed by anti–mouse Alexa-488–conjugated IgG (green). Bars represent 2 μm.
Immunofluorescence analysis of STEVOR protein expression in GIEs. (A) Analysis of STEVOR protein expression in fixed stages II to V GIE preparations. GIEs were acetone-fixed and costained with mouse anti-S2 (green) and rabbit anti-Pfg27 (red) followed by anti–mouse Alexa-488– and anti–rabbit Alexa-594–conjugated IgG. Parasite nuclei were stained with DAPI (blue). Bright-field (BF) and merge images are shown. Bars represent 2 μm. (B) Analysis of live GIEs immunostained with rabbit anti-S2 (green) specifically recognizing native STEVOR on the surface of stages III and IV GIEs. Bound antibody was detected with anti–rabbit Alexa-488–conjugated IgG. BF, nuclear staining (DAPI, blue), and merge images are shown. (C) Immunofluorescence analysis of STEVOR expression in fixed stages II to V-GIEs. GIEs were methanol-fixed and stained with a pool of mouse polyclonal antibodies directed against STEVOR proteins followed by anti–mouse Alexa-488–conjugated IgG (green). Bars represent 2 μm.
STEVOR expression and localization contribute to GIE deformability
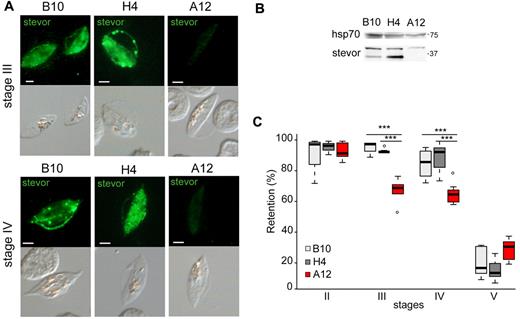
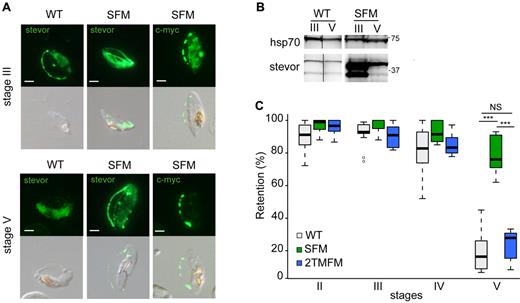
To test this hypothesis, stage-specific GIE deformability was analyzed in gametocytes defective in the expression or, on the contrary, overproducing STEVOR proteins. In the first set of experiments, the NF54-derived clone A12, in which mRNA production of all stevor genes is down-regulated during asexual development,16 was used to produce gametocytes. Immunoblotting and immunostaining with anti-STEVOR polyclonal sera on methanol-fixed GIEs from clone A12 indicated that STEVOR expression was significantly decreased in A12 gametocytes compared with those produced by the sibling B10 and H4 clones (Figure 4A-B; supplemental Figure 4). Microsphiltration experiments on immature GIEs from these clones showed similar retention rates in stage II but revealed a significant decrease in retention rates in A12 compared with B10 and H4 in stage III (68.2% vs 96% and 93.8%, P = .0051 and .0025) and stage IV GIEs (65.8% vs 85.3% and 89%, P = .0086 and .0041). Mature GIEs showed, as expected, similar low retention rates in all clones (Figure 4C). In the second set of experiments, STEVOR overproduction in gametocytes was achieved in the transgenic parasite line STEVOR-Flag-c-myc (SFM), which overexpresses a c-myc–tagged copy of the PFF1550w stevor gene driven by the hrp3 promoter.15 Immunoblotting showed that STEVORs were more abundant in SFM than in wild-type gametocytes (Figure 5B). Importantly, immunostaining with anti-STEVOR polyclonal sera and an anti–c-myc mAb on methanol-fixed GIE in the SFM line revealed that the overexpressed STEVOR protein was partly localized at the infected erythrocyte membrane also in stage V GIEs (Figure 5A). Microsfiltration analysis of synchronous wild-type and SFM gametocytes showed that retention rates were similar at stages II and III and slightly higher in SFM parasites at stage IV (P = .03). However, the retention rate of stage V GIEs was substantially higher in SFM than in wild-type parasites (78% vs 23%, P = .00001), suggesting that STEVOR overexpression and its persistence at the erythrocyte membrane in stage V GIE significantly impaired the increase in deformability associated with wild-type GIE maturation (Figure 5C). To exclude that retention of SFM stage V was the result of delayed sexual maturation, stage V gametocytes were tested for their exflagellation efficiency. Result was that SFM stage V exflagellated with the same kinetics and efficiency as wild-type gametocytes (not shown). To control for possible artifactual increase in the rigidity of SFM stage V GIEs resulting from transgene overexpression, gametocytes overproducing a c-myc-tagged member of the Pfmc-2TM family, obtained from the transgenic line 2TMFM, were analyzed. Retention rates for 2TMFM GIEs were similar to those of the wild-type line at all stages, indicating that the increased retention rates of mature SFM GIEs were specifically linked to STEVOR overexpression (Figure 5C).
Decrease in STEVOR expression is associated with an increase in deformability of immature GIEs. (A) Immunofluorescence analysis of STEVOR expression in stage III and stage IV GIEs from the B10, H4, and A12 clones. GIEs were stained with a pool of mouse polyclonal antibodies directed against STEVOR proteins followed by anti–mouse Alexa-488–conjugated IgG. Signal intensity was analyzed by ImageJ Version 6 on at least 30 pictures taken under identical exposition conditions for each clone. Bars represent 2 μm. (B) Western blot analysis of STEVOR expression in stage III and stage V GIEs from the wild-type and the SFM parasites. Immunoblots were probed with a pool of mouse polyclonal antibodies directed against STEVOR proteins and with a mAb directed against HSP70. (C) Retention in microsphilters of stages II, III, IV, and V GIEs from the B10 (light gray), H4 (dark gray), and A12 (red) clones. ***Highly significant differences in retention rates (P < .01). Outliers are shown as open circles.
Decrease in STEVOR expression is associated with an increase in deformability of immature GIEs. (A) Immunofluorescence analysis of STEVOR expression in stage III and stage IV GIEs from the B10, H4, and A12 clones. GIEs were stained with a pool of mouse polyclonal antibodies directed against STEVOR proteins followed by anti–mouse Alexa-488–conjugated IgG. Signal intensity was analyzed by ImageJ Version 6 on at least 30 pictures taken under identical exposition conditions for each clone. Bars represent 2 μm. (B) Western blot analysis of STEVOR expression in stage III and stage V GIEs from the wild-type and the SFM parasites. Immunoblots were probed with a pool of mouse polyclonal antibodies directed against STEVOR proteins and with a mAb directed against HSP70. (C) Retention in microsphilters of stages II, III, IV, and V GIEs from the B10 (light gray), H4 (dark gray), and A12 (red) clones. ***Highly significant differences in retention rates (P < .01). Outliers are shown as open circles.
Stevor overexpression is associated with a decrease in deformability of mature GIEs. (A) Immunofluorescence analysis of STEVOR expression in stage III and stage V GIEs from the wild-type (WT) and the SFM parasites. GIEs were stained with a pool of mouse polyclonal antibodies directed against STEVOR proteins or with anti–c-myc mAb followed by anti–mouse Alexa-488–conjugated IgG. Bars represent 2 μm. (B) Western blot analysis of STEVOR expression in stage III and stage V GIEs from the WT and the SFM parasites. Immunoblots were probed with a pool of mouse polyclonal antibodies directed against STEVOR proteins and with a mAb directed against HSP70. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Retention in microsphilters of stages II, III, IV, and V GIEs from different WT P falciparum clonal lines (WT representing B10, H4, and 3D7GFP clones, in gray), from the SFM parasite line overexpressing a c-myc–tagged copy of the PFF1550w stevor gene (green) and the 2TMFM parasite line overexpressing a c-myc-tagged copy of the PFA0680c Pfmc-2TM gene (blue). ***Highly significant differences in retention rates (P < .01). Outliers are shown as open circles. NS indicates not significant.
Stevor overexpression is associated with a decrease in deformability of mature GIEs. (A) Immunofluorescence analysis of STEVOR expression in stage III and stage V GIEs from the wild-type (WT) and the SFM parasites. GIEs were stained with a pool of mouse polyclonal antibodies directed against STEVOR proteins or with anti–c-myc mAb followed by anti–mouse Alexa-488–conjugated IgG. Bars represent 2 μm. (B) Western blot analysis of STEVOR expression in stage III and stage V GIEs from the WT and the SFM parasites. Immunoblots were probed with a pool of mouse polyclonal antibodies directed against STEVOR proteins and with a mAb directed against HSP70. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Retention in microsphilters of stages II, III, IV, and V GIEs from different WT P falciparum clonal lines (WT representing B10, H4, and 3D7GFP clones, in gray), from the SFM parasite line overexpressing a c-myc–tagged copy of the PFF1550w stevor gene (green) and the 2TMFM parasite line overexpressing a c-myc-tagged copy of the PFA0680c Pfmc-2TM gene (blue). ***Highly significant differences in retention rates (P < .01). Outliers are shown as open circles. NS indicates not significant.
Discussion
We investigated changes in deformability and filterability of erythrocytes infected with immature and mature gametocytes of P falciparum. Our results establish that immature GIEs are poorly deformable and that a switch in deformability occurs at the transition from immature to mature GIEs. In human infectionsm this developmental step coincides with the appearance of mature gametocyte in circulation after release from their yet unknown sequestration sites in the body, and with their restored ability to circulate in blood and to cross narrow capillaries and splenic slits. Microsphiltration results reported here establish that erythrocytes infected by immature gametocytes exhibit high retention rates comparable with those of mature asexual stages. In contrast, mature GIEs displayed deformability properties similar to those of erythrocytes infected with the freely circulating ring stage parasites.14 The independent technical approach of ektacytometry in our work and in a recent report31 also showed that mature GIEs are significantly more deformable than immature GIEs, confirming the microsphiltration experiments. These results are altogether consistent with the need for mature gametocytes to cross the splenic slits to circulate, a prerequisite to be ingested by Anopheles and ensure transmission. We show here that the STEVOR family plays a role in this process, as immature gametocytes underexpressing such proteins display an increased deformability compared with wild-type immature stages, and gametocytes overexpressing STEVORs remain poorly deformable also at the mature stage.
The integration in the present work of observations on whole GIE mechanical properties, gametocyte morphology, and dynamic association of parasite molecules with the erythrocyte membrane provides the first clues on the mechanisms governing the developmental changes in GIE deformability. Changes in mechanical properties of P falciparum-infected erythrocytes result from the combination of presence of the intracellular parasite and parasite-induced modifications of its erythrocyte host. Erythrocyte deformability is determined by 3 factors: the surface area and volume of the cell, the cytoplasmic viscosity, and the membrane viscoelasticity.32 The altered morphology of GIEs fixed inside the microsphere matrix shows that mature gametocytes themselves are, unlike the immature stages, highly stretched within the erythrocyte, suggesting an increase in intrinsic parasite deformability during sexual maturation. A process probably responsible for such a modification is the disassembly of the microtubular subpellicular network subtending the trilaminar membrane structure in the transition from stage IV to stage V gametocytes.5 Recent cryo x-ray tomography analysis revealed that the surface area and the volume of GIEs remain roughly constant during gametocyte development,27 suggesting limited impact of these factors on the observed switch in deformability at the transition from stage IV to stage V. The same study also showed that approximately 70% of the erythrocyte hemoglobin is digested during gametocytogenesis. As this decrease is progressive throughout sexual development, it is unlikely that changes in GIE cytoplasmic viscosity entirely account for the abrupt switch in deformability at the transition between stage IV and stage V GIE. It is therefore plausible that decreased membrane viscoelasticity contributes to stiffness of immature GIEs, which, based on observations with asexual stages,33 is probably a consequence of mediation by parasite-encoded proteins associating with the erythrocyte membrane skeleton.
The findings that STEVORs associate with the erythrocyte membrane in immature GIEs and that their disappearance from that site coincides with the increased deformability of mature GIEs suggest that these transmembrane proteins significantly contribute to the decrease in membrane viscoelasticity of immature GIEs. The mechanisms underlying STEVOR-mediated stiffness remain elusive. A possible interaction with erythrocyte cytoskeletal proteins may induce spectrin cross-linking, as proposed for the plasmodial proteins KAHRP and PfEMP3 exported in asexual stages.34-36 Deassociation from the erythrocyte membrane in mature stages may result from proteolytic cleavage or conformational changes on posttranslational modifications. Such events are probably responsible for the failure of our antibodies to detect STEVOR association with the erythrocyte membrane in mature GIEs, although this was observed using a different set of antibodies raised to small peptides.12
Which are the sites of P falciparum gametocyte sequestration in the human body, how immature gametocytes hide in such sites for almost 10 days, how do mature stages regain access to blood circulation, and which are the mechanisms ensuring their persistence in peripheral circulation for several days are still major unanswered fundamental questions in the biology of malaria parasites. Although a mechanistic hypothesis on gametocyte sequestration is virtually impossible without the identification of the sites of sexual stage sequestration in vivo, our results allow us to speculate that immature GIE stiffening and the increase in GIE deformability at gametocyte maturation may be functionally linked with the dynamics of this phenomenon. Once sequestration is established by the round, trophozoite-like stage I gametocytes through yet elusive mechanism(s), we hypothesize that mechanical retention may significantly contribute to maintenance of sequestration throughout the ensuing maturation process of GIEs. This is consistent with the fact that PfEMP1 and knob structures are absent from the surface of stage II to stage V GIEs5,8 and that immature GIEs show only weak, if any, binding interactions with endothelial host cells.7,8,11 Observations from postmortem and ex vivo specimens and from rare clinical cases, altogether indicating that bone marrow and spleen are the organs where immature gametocytes are more readily found,2,3,37 suggest that mechanical retention of immature gametocytes may be favored in such tissues, characterized by an open and slow blood circulation. A role of adhesins in gametocyte sequestration cannot, however, be presently excluded. Recent findings that STEVORs have adhesive properties and are involved in rosetting in asexual stages (M.N. et al, unpublished results) and our observation that STEVORs is accessible to antibodies on the GIE surface up to stage IV may suggest a more direct role of such proteins in GIE cytoadhesion, requiring further studies.
Our results suggest that the high deformability specifically characterizing stage V GIEs may facilitate release from sequestration sites. Importantly, we propose that such a property restores the ability of mature GIEs to cross narrow apertures and allows them to escape mechanical retention in the spleen, thus contributing to their sustained circulation in the peripheral blood where they are available to the insect vector for several days. We propose that interventions targeting these processes, thereby reducing the ability of mature GIEs to circulate, open novel avenues in the present strategies to reduce parasite transmission.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank François Lacoste (Fondation Ackerman–Fondation de France) for fruitful discussions and financial support.
M.T. and P.A. were supported by the EU FP7 Marie Curie ITN InterMalTraining (contract PITN-GA-2008-215281). O.M.-P. and P.A. were supported by the FP7 NoE EVIMalaR (contract 242095). M.N. and P.R.P. were supported by the BioMedical Research Council Singapore. C.L., K.D.V., G.M., P.B., and O.M.-P. were supported by Région Ile de France, the Fondation Symphasis, and the National Institutes of Health Project “Mechanisms of Erythrocytic Infection and Anemia in Malaria” (Award 5P01HL078826-06).
National Institutes of Health
Authorship
Contribution: M.T., M.N., G.D., S.P., P.A.N., F.S., and C.L. performed experiments; M.T., M.N., P.A., and C.L. analyzed results and made figures; E.B. performed statistical analysis; A.K., G.M., M.H., K.D.V., R.W.S., O.M.-P., and P.B. analyzed data and contributed vital new reagents or analytical tools; M.T., M.N., P.H.D., P.R.P., P.A., and C.L. designed the research; and M.T., P.A., and C.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pietro Alano, Dipartimento di Malattie Infettive, Parassitarie e Immunomediate, Istituto Superiore di Sanità, Rome, Italy; e-mail: alano@iss.it; and Catherine Lavazec, Département de Parasitologie Mycologie; Unité d'Immunologie Moléculaire des Parasites, CNRS, URA 2581, Institut Pasteur, 28 rue du Docteur Roux, Paris, France; e-mail: clavazec@pasteur.fr.
References
Author notes
M.T. and M.N. contributed equally to this study.