Abstract
The SHIELD program for Hodgkin lymphoma in patients 60 years of age or older, prospectively evaluated clinical features and outcome in a large patient cohort (n = 175). The central element was a phase 2 study of VEPEMB chemotherapy (n = 103, median age 73 years) incorporating comorbidity assessment. A total of 72 other patients were treated off-study but registered prospectively and treated concurrently with: ABVD (n = 35); CLVPP (n = 19), or other (n = 18). Of VEPEMB patients, 31 had early-stage disease (stage 1A/2A) and received VEPEMB 3 times plus radiotherapy. Median follow-up was 36 months. Complete remission (CR) rate (intention-to-treat) was 74% and 3-year overall survival (OS) and progression-free survival (PFS) were 81% and 74%, respectively. A total of 72 patients had advanced-stage disease (stage 1B/2B/3 or 4) and received VEPEMB 6 times. CR rate was 61% with 3-year OS and PFS of 66% and 58%, respectively. Of patients achieving CR, 13% with early-stage and 5% with advanced-stage disease progressed. Overall treatment-related mortality was 7%. In patients treated with curative intent with VEPEMB, ABVD, and CLVPP (n = 157), CR linked to several factors in univariate analysis. In a Cox regression model only, obtaining CR remained significant for OS and CR plus comorbidity and age for PFS. RS-EBV status had no significant effect on outcome.
1. Introduction
In the 1990s, the Scotland and Newcastle Lymphoma Group (SNLG) undertook a population-based review of Hodgkin lymphoma (HL) in patients 60 years of age or older.1 We found that clinical outcomes had not improved over the previous decade and lagged behind results reported for younger patients. SNLG population-based data indicated that the incidence of HL in those 60 years of age or older was 5 cases/million total population per year and accounted for 20% of all HL cases. In those 60 to 70 years of age, clinical outcome was found to be acceptable in early-stage disease but was very poor in advanced-stage HL. Results in those older than 70 years were particularly poor, irrespective of stage. Comprehensive reviews of all published studies in the elderly have confirmed these outcomes and called for new research initiatives.2-4
In 2001, the SNLG took the lead in developing alternative ways of studying the problem of HL in the elderly. The process began with workshop discussions at the Cologne Hodgkin Lymphoma meeting and the Malignant Lymphoma meeting in Lugano.5 A consensus emerged at these meetings regarding the need for further study and data collection. There was, however, no consensus on therapeutic approaches, except to affirm that ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), the gold standard for chemotherapy for younger patients,6 was probably too toxic for many older patients, particularly those older than 70 years. Given the lack of a standard therapy, it was clear that a randomized trial would not be immediately feasible. It was agreed that individual international groups would set up phase 2 studies aimed at generating an effective treatment regimen with acceptable toxicity. In addition, the groups agreed to further evaluate, on a prospective basis, the challenge of trial organization for patients in this age group.
Within the German Hodgkin Study Group (GHSG), these questions generated 2 phase 2 studies with modified chemotherapy regimens. The first, BACOPP (bleomycin, doxorubicin, cyclophosphamide, vincristine, prednisolone, and procarbazine), was a modification of the BEACOPP regimen (as BACOPP but with the addition of etoposide and with all drugs administered at higher doses) used in younger patients.7 The second was the gemcitabine-containing PVAG regimen (prednisone, vincristine, adriamycin, and gemcitabine).8
In the United Kingdom, a decision was made to adopt and reassess prospectively the VEPEMB schedule (vinblastine, cyclophosphamide, prednisolone, procarbazine, etoposide, mitoxantrone, and bleomycin) developed and evaluated by the Italian Lymphoma Group,9 as this group had the best published outcome data at that time, derived from a study of more than 100 patients.
This approach led to the development of the SHIELD (Study of Hodgkin in the Elderly/Lymphoma Database) program. This was a prospective study designed with 2 components: (1) a prospective phase 2 trial of VEPEMB and (2) a prospective registration study of patients not treated as part of the VEPEMB study. This included patients too frail to receive standard therapies2,5 and patients managed with curative intent using alternative treatment regimens. In addition, the SHIELD study aimed to use prospective Web-based data collection for patient registration, to assess its value as a generic study tool in “orphan diseases” in oncology and to introduce objective assessment of comorbidity and other functional parameters.10 This report details the outcome of the 175 United Kingdom patients entered onto both components of the SHIELD program.
Methods
Patients and study design
The SHIELD program was badged (National Institute of Health Research 1328) as a United Kingdom National Institute of Health Research protocol after peer review. The protocol and ethical regulatory documentation is available on the program's Web site (www.shieldstudy.co.uk).
Eligible patients at participating centers were those 60 years of age or older at the time of diagnosis of HL. Patients could be consented into either the phase 2 study of VEPEMB or into the registration arm of the study. Recruiting centers were encouraged to recruit to the study all patients presenting in their center, to allow, as far as possible, a population-representative group to be evaluated. Initial evaluation involved a formalized assessment of comorbidity (Table 1). If patients were designated as “nonfrail,” on the comorbidity assessment they were eligible for the phase 2 VEPEMB protocol. Those designated as “frail” were eligible for entry into the registration arm of the study and were treated at their physician's discretion. The registration arm of the study was also open to “nonfrail” patients who could be treated at the discretion of the treating physician with any regimen of their choice.
Modified ACE-27 comorbidity rating scheme used in SHIELD study
| Comorbidity scale . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|---|
| Instructions: | |||||
| 1. Select a grade for each of the systems. | |||||
| 2. Press the calculate button to add up the number of individual grades. | |||||
| 3. Use the totals to work out which answer to use (frail/not frail). | |||||
| All grade 1 (not frail) | |||||
| All grade 2 or less (not frail) | |||||
| Two grade 3 or higher (not frail) | |||||
| More than three grade 3 or at least one grade 4/5 (“frail”) | |||||
| Grade key | |||||
| 1. No disability | |||||
| 2. Low disability (it does not interfere with normal activities; treatment is optional; very good prognosis) | |||||
| 3. Mild disability (it interferes with normal activities; treatment is needed; good prognosis) | |||||
| 4. Severe disability (disabling, urgent treatment is needed; poor prognosis) | |||||
| 5. Very severe disability (it can be fatal; emergency treatment is needed; danger list) | |||||
| System | |||||
| Heart (heart only) | ○ | ○ | ○ | ○ | ○ |
| Hypertension (hypertension severity is considered) | ○ | ○ | ○ | ○ | ○ |
| Vascular (both venous and artery districts) | ○ | ○ | ○ | ○ | ○ |
| Diabetes | ○ | ○ | ○ | ○ | ○ |
| Respiratory | ○ | ○ | ○ | ○ | ○ |
| Gastrointestinal | ○ | ○ | ○ | ○ | ○ |
| Liver | ○ | ○ | ○ | ○ | ○ |
| Kidney | ○ | ○ | ○ | ○ | ○ |
| Other genitourinary | ○ | ○ | ○ | ○ | ○ |
| Bone and muscle | ○ | ○ | ○ | ○ | ○ |
| Nervous system (central and peripheral, dementia is excluded) | ○ | ○ | ○ | ○ | ○ |
| Eyes and ENT (eyes, ears, nose, larynx) | ○ | ○ | ○ | ○ | ○ |
| Endocrine system and metabolism (diabetes excluded) | ○ | ○ | ○ | ○ | ○ |
| Calculate: | |||||
| If “frail,” not eligible for VEPEMB study | 1's | 2's | 3's | 4's | 5's |
| Total | ○ | ○ | ○ | ○ | ○ |
| Comorbidity scale . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|---|
| Instructions: | |||||
| 1. Select a grade for each of the systems. | |||||
| 2. Press the calculate button to add up the number of individual grades. | |||||
| 3. Use the totals to work out which answer to use (frail/not frail). | |||||
| All grade 1 (not frail) | |||||
| All grade 2 or less (not frail) | |||||
| Two grade 3 or higher (not frail) | |||||
| More than three grade 3 or at least one grade 4/5 (“frail”) | |||||
| Grade key | |||||
| 1. No disability | |||||
| 2. Low disability (it does not interfere with normal activities; treatment is optional; very good prognosis) | |||||
| 3. Mild disability (it interferes with normal activities; treatment is needed; good prognosis) | |||||
| 4. Severe disability (disabling, urgent treatment is needed; poor prognosis) | |||||
| 5. Very severe disability (it can be fatal; emergency treatment is needed; danger list) | |||||
| System | |||||
| Heart (heart only) | ○ | ○ | ○ | ○ | ○ |
| Hypertension (hypertension severity is considered) | ○ | ○ | ○ | ○ | ○ |
| Vascular (both venous and artery districts) | ○ | ○ | ○ | ○ | ○ |
| Diabetes | ○ | ○ | ○ | ○ | ○ |
| Respiratory | ○ | ○ | ○ | ○ | ○ |
| Gastrointestinal | ○ | ○ | ○ | ○ | ○ |
| Liver | ○ | ○ | ○ | ○ | ○ |
| Kidney | ○ | ○ | ○ | ○ | ○ |
| Other genitourinary | ○ | ○ | ○ | ○ | ○ |
| Bone and muscle | ○ | ○ | ○ | ○ | ○ |
| Nervous system (central and peripheral, dementia is excluded) | ○ | ○ | ○ | ○ | ○ |
| Eyes and ENT (eyes, ears, nose, larynx) | ○ | ○ | ○ | ○ | ○ |
| Endocrine system and metabolism (diabetes excluded) | ○ | ○ | ○ | ○ | ○ |
| Calculate: | |||||
| If “frail,” not eligible for VEPEMB study | 1's | 2's | 3's | 4's | 5's |
| Total | ○ | ○ | ○ | ○ | ○ |
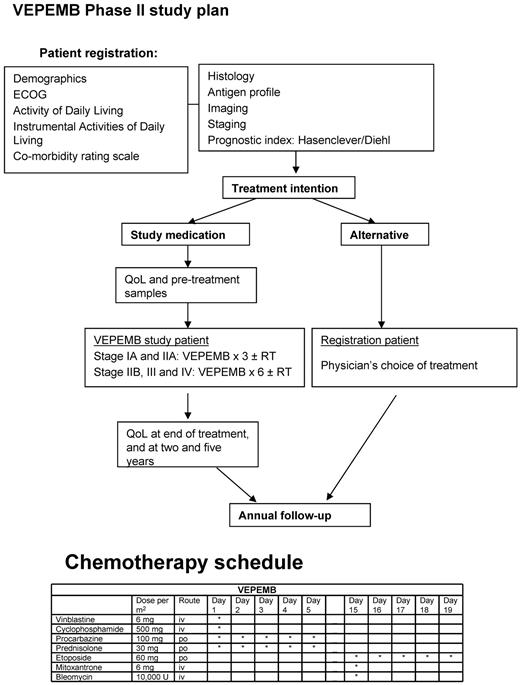
The protocol for the phase 2 study of VEPEMB for patients with both early-stage (stage 1A and 2A) and advanced-stage (stages 1B, 2B, 3A/B, and 4A/B) HL is outlined in Figure 1. G-CSF was used routinely to support blood counts. Co-trimoxazole (960 mg 3 times per week) was recommended as prophylaxis for all patients.
The scheme of treatment for patients on the SHIELD program, including drug doses and schedule for VEPEMB.
The scheme of treatment for patients on the SHIELD program, including drug doses and schedule for VEPEMB.
At recruitment, routine hematologic and blood chemistry parameters, including lactate dehydrogenase and albumin, were measured. Patients were staged radiologically by CT scanning. The study predated the routine availability of FDG-PET scanning. Bone marrow biopsy was recommended for all patients. Individual patient risk scores were calculated using the Hasenclever/Diehl score. Comorbidity was assessed using a modified ACE-27 comorbidity scale (Table 1).11 Activities of daily living (ADL) and instrumental activities of daily living (IDL) were assessed using standardized scoring systems.12,13
Central pathology review was requested for all biopsies from patients entered into the VEPEMB phase 2 trial. Original diagnostic slides and at least 10 unstained sections and/or a representative paraffin block were requested for each case. Minimum requirements for review were as follows: a hematoxylin and eosin section and immunohistochemically stained slides for CD3, CD15, CD20, and CD30. The presence or absence of EBV was assessed by immunohistochemistry (LMP1) and/or in situ hybridization. Cases were subclassified according to WHO criteria.14 Where central pathology review was not possible, histologic categorizations and EBV status assigned by the referring center are reported.
Follow-up was to 12 months after the recruitment of the last patient to the VEPEMB study or the date the patient was last known to be alive. Median follow-up was 36 months (range, 12-80 months). Overall survival (OS) was measured from date of histologic diagnosis until date of death. For disease-specific survival, patients were censored at the time of death if death was unrelated to HL or its treatment. Progression-free survival (PFS) was calculated from date of histologic diagnosis to first disease progression, with patient withdrawal, death from any cause, nonresponse (NR), and partial response all regarded as events. Complete remission (CR) was defined as no evidence of disease on repeat scanning after treatment. Partial remission (PR) was defined as a reduction in tumor volume of at least 50% compared with pretreatment imaging. NR was defined as failure to achieve PR. Progressive disease (PD) was defined as increased size of the tumor despite therapy.
Data collection
One goal of the SHIELD program was to create a study platform to allow a national/international program for elderly patients to be conducted online, with a minimal staff involved at the study coordinating center (Chief Investigator and Trial Coordinator). This methodology has the potential to provide a practical new approach for the study of “orphan” diseases.10 To this end, a purpose-built secure software package was developed (Power-Trial). This system allowed all aspects of study management to be performed online to conform to Good Clinical Practice and FDA-Code of Federal Regulations 11 part 2 requirements. The only elements requiring paper were the reporting of serious adverse events by fax. All data could be viewed in real time by the chief investigator and trial manager. Site-specific data could be viewed by the individual site study coordinators and principal investigators.
Statistical methods
Means and SDs were used to summarize normally distributed variables, and medians and quartiles were used to summarize variables with a skew distribution. The t test was used to compare means of normally distributed variables in 2 groups and the Wilcoxon-Sign test to compare medians for skew data. Proportions across categories were compared using χ2 tests, and proportions across ordinal categories were compared using the χ2 trend test. The Fisher exact test was used in a 2 × 2 table when 1 or more counts was less than 5. Survival patterns in 2 or more groups were compared using the log-rank test.
Results
In the United Kingdom, 53 centers obtained approval for participation. Ten of these centers did not recruit. Of the remaining 43 centers, 4 recruited more than 10 patients, 6 recruited 5 to 9 patients, and 33 recruited 1 to 4 patients.
A total of 175 patients were recruited. A total of 116 of 175 (66%) consented to entry into the VEPEMB phase 2 study. Thirteen of 116 (11%) were found to be frail on the comorbidity assessment and were treated at physician discretion, and patient data were recorded within the registration study. A total of 59 of 175 (34%) were recruited directly into the registration study at the outset, and treatment was designated by the treating physician. Figure 2 summarizes treatment strategies for all 175 recruited patients. A total of 103 patients were designated nonfrail and thus eligible for VEPEMB on the trial arm of the program.
Clinical outcome summary. (A) A flow diagram indicating progress of patients treated on the VEPEMB phase 2 study. Patient responses to chemotherapy are shown. Thirteen patients failed comorbidity assessment (designated “frail”) and were not eligible for the VEPEMB study. Comparison of the “frail” group with all VEPEMB indicates inferior treatment response (P < .001, Fisher exact test). A similar effect was evident on survival (P < .001, log-rank test). AS indicates advanced-stage disease; ES, early-stage disease; and NA, not assessable for response. (B) Choice of therapy for patients treated on the registration arm of the study. AS indicates advanced-stage disease; ES, early-stage disease; and NA, not assessable for response.
Clinical outcome summary. (A) A flow diagram indicating progress of patients treated on the VEPEMB phase 2 study. Patient responses to chemotherapy are shown. Thirteen patients failed comorbidity assessment (designated “frail”) and were not eligible for the VEPEMB study. Comparison of the “frail” group with all VEPEMB indicates inferior treatment response (P < .001, Fisher exact test). A similar effect was evident on survival (P < .001, log-rank test). AS indicates advanced-stage disease; ES, early-stage disease; and NA, not assessable for response. (B) Choice of therapy for patients treated on the registration arm of the study. AS indicates advanced-stage disease; ES, early-stage disease; and NA, not assessable for response.
Pathology
Material was received for central pathology review for 87 of 103 VEPEMB trial patients; classic HL was confirmed in all cases. Histologic case distribution was: 18 classic HL not further classifiable (14 confirmed by central review), 47 nodular sclerosis HL (42 confirmed by central review), 34 mixed cellularity HL (29 confirmed by central review), and 4 lymphocyte-rich classic HL (2 confirmed by central review).
EBV status of RS cells
Sufficient material was available to confirm the EBV status of the Reed-Sternberg cells by central review in 84 of 103, and information provided by the referring center was available for a further 10 cases. When all cases were considered, 51 of 94 were EBV-positive, 8 of 15 classic HL not further classifiable, 20 of 43 nodular sclerosis HL, 22 of 33 mixed cellularity HL, and 1 of 3 lymphocyte-rich classic HL.
Outcomes
Phase 2 VEPEMB study patients.
The median age of VEPEMB-treated patients was 73 years (range, 61-85 years). Response was evaluable for 90 of 103 patients. Three patients died of sepsis before first clinical assessment, 1 patient died of rapidly progressive HL, and 4 additional patients died during the first 2 cycles of chemotherapy (Table 2). Five patients (3 with early-stage and 2 with advanced-stage disease) withdrew consent to the trial or were withdrawn by their physician, before receiving 2 courses of VEPEMB, and were given alternative treatments. Four received radiotherapy alone, and CLVPP was used in 1 case. Although it was not possible to assess response in these 13 patients, they are included in intention-to-treat (ITT) outcome analysis.
Serious adverse events: SHIELD study
| Type of event . | WHO grade 1 or 2 . | WHO grade 3 or 4 . | Comments . |
|---|---|---|---|
| Infective | |||
| Neutropenic sepsis, organ not specified | 5 | 16 | 3 fatal |
| Infective, organ specified | 9 | 3 | |
| Pyrexia unknown, origin presumed infective (not neutropenic) | 11 | 0 | |
| Hematologic | 4 | 13 | Neutropenia (12), neutropenia and thrombocytopenia (2), anemia (3) |
| Cardiovascular | 3 | 5 | DVT (2), myocardial infarct (2, one fatal), arrhythmia (1), chest pain (2), sudden death presumed cardiac (1) |
| Nervous system | 3 | 1 | Stroke (2, one fatal), motor weakness (1), epilepsy (1) |
| Pulmonary (noninfective) | 2 | 0 | Pulmonary fibrosis (bleomycin) (1), SOB, ? cause (1) |
| Gastrointestinal | 7 | 8 | |
| Renal | 1 | 0 | Hydronephrosis |
| Bone | 1 | 0 | |
| Cancer | 0 | 3 | AML (1), rectal cancer (1), carcinoid (1); 3 cancers reported as SAE, others discussed in text occurred in follow-up period |
| Metabolic | 1 | 0 | Hyponatremia |
| ENT | 1 | 0 | Tinnitus |
| Total | 48 | 49 |
| Type of event . | WHO grade 1 or 2 . | WHO grade 3 or 4 . | Comments . |
|---|---|---|---|
| Infective | |||
| Neutropenic sepsis, organ not specified | 5 | 16 | 3 fatal |
| Infective, organ specified | 9 | 3 | |
| Pyrexia unknown, origin presumed infective (not neutropenic) | 11 | 0 | |
| Hematologic | 4 | 13 | Neutropenia (12), neutropenia and thrombocytopenia (2), anemia (3) |
| Cardiovascular | 3 | 5 | DVT (2), myocardial infarct (2, one fatal), arrhythmia (1), chest pain (2), sudden death presumed cardiac (1) |
| Nervous system | 3 | 1 | Stroke (2, one fatal), motor weakness (1), epilepsy (1) |
| Pulmonary (noninfective) | 2 | 0 | Pulmonary fibrosis (bleomycin) (1), SOB, ? cause (1) |
| Gastrointestinal | 7 | 8 | |
| Renal | 1 | 0 | Hydronephrosis |
| Bone | 1 | 0 | |
| Cancer | 0 | 3 | AML (1), rectal cancer (1), carcinoid (1); 3 cancers reported as SAE, others discussed in text occurred in follow-up period |
| Metabolic | 1 | 0 | Hyponatremia |
| ENT | 1 | 0 | Tinnitus |
| Total | 48 | 49 |
DVT indicates deep vein thrombosis; SOB, shortness of breath; and AML, acute myeloid leukemia.
Based on ITT analysis, 23 of 31(74%) of early-stage patients achieved CR. For advanced-stage patients, CR rate was 44 of 72 (61%). At a median follow-up of 3 years, there was a striking maintenance of CR with only 3 (13%) early-stage and 2 (5%) advanced-stage patients relapsing. For stage 3 and 4 VEPEMB-treated patients specifically, OS and PFS at 36 months were 62% and 53%, respectively. There were 7 deaths in remission during follow-up. In 4 cases, death was the result of other malignancies, including 1 case of acute myeloid leukemia occurring 2 years after therapy. Thus, 3 septic deaths (3%) and 4 malignant deaths (4%) give a 7% overall treatment-related mortality. Other causes of death were vascular events (n = 2) and bowel perforation (n = 1).
By contrast, of the 18 patients who obtained PR after VEPEMB therapy (2 with early and 16 with advanced-stage disease), 10 died of progressive HL, and 3 died of other causes, having failed all salvage approaches with a median OS of 18 months. At the time of analysis, 3 patients are in continuing PR after second-line therapy. Only 2 patients attained CR with second-line chemotherapy; one with ESHAP then BEAM autologous hematopoietic stem cell transplantation, the other with oral PECC (prednisolone, etoposide, chlorambucil, and lomustine). These remissions were continuing at 18 and 24 months, respectively. Of the 5 patients with PD, 3 died of progression, 1 entered PR/CRu, continuing at 31months, after modified ABVD, and 1 attained CR, which continues at 18 months, after oral PECC and rituximab.
Toxicity
Three septic deaths were recorded. One occurred only 3 days after patient registration and 1 day after therapy. The other 2 deaths were clearly therapy-related events in the neutropenic phase. Incidence of infective therapy-related death was 3 of 103 (3%). All septic deaths occurred in patients with ongoing active HL. All patients treated with VEPEMB demonstrated more than grade 2 hematotoxicity and required G-CSF support as per protocol. Details of recorded serious adverse events during therapy are shown in Table 2. One VEPEMB patient developed pulmonary fibrosis attributed to bleomycin, an incidence of 1%.
Chemotherapy delivery
Formal dose intensity measurements were not assessable for VEPEMB patients because of missing data in a proportion of patients. Course completion was recorded for 95% of patients, and dose reductions were recorded as necessary in 67% of patients. Both parameters are significantly associated with rate of CR. These data are shown in Table 3.
Effect of course completion and dose reduction on CR rates in VEPEMB-treated patients
| . | Not CR, no. . | CR, no. . | CR, % . | Total no. . |
|---|---|---|---|---|
| Course completion | ||||
| Not completed | 19 | 15 | 44 | 34 |
| Completed | 15 | 49 | 76 | 64 (P = .001) |
| Data incomplete | 0 | 0 | 0 | 5 |
| Total | 34 | 64 | 65.3 | 103 |
| Dose reduction | ||||
| No | 11 | 34 | 75.6 | 45 |
| Yes | 12 | 12 | 50 | 24 (P = .03) |
| Data incomplete | 0 | 0 | 0 | 34 |
| Total | 23 | 46 | 66.7 | 103 |
| . | Not CR, no. . | CR, no. . | CR, % . | Total no. . |
|---|---|---|---|---|
| Course completion | ||||
| Not completed | 19 | 15 | 44 | 34 |
| Completed | 15 | 49 | 76 | 64 (P = .001) |
| Data incomplete | 0 | 0 | 0 | 5 |
| Total | 34 | 64 | 65.3 | 103 |
| Dose reduction | ||||
| No | 11 | 34 | 75.6 | 45 |
| Yes | 12 | 12 | 50 | 24 (P = .03) |
| Data incomplete | 0 | 0 | 0 | 34 |
| Total | 23 | 46 | 66.7 | 103 |
The majority of dose adjustments were made in patients who subsequently failed to attain CR, although 10 patients treated with a reduced number of chemotherapy courses have achieved sustained CR. Dose adjustments were principally made in response to recurrent neutropenia or infective episodes.
Prognostic factors for VEPEMB-treated cohort (n = 103)
Statistical analysis of factors associated with CR in VEPEMB patients demonstrated significant association with ECOG status (0 or 1 vs 2 or 3, P = .021), serum albumin at diagnosis (normal vs abnormal, P = .01), treatment course completion (completion vs noncompletion, P = .001), and dose reduction (reduction vs nonreduction, P = .03).
Nonsignificant factors were, age, stage, sex, histologic subtype, Hasenclever score, hemoglobin, lactate dehydrogenase, lymphocyte count, and number of nodal sites involved. Reed-Sternberg cell EBV status was not significantly associated with CR rate or survival (P = .716).
Comorbidity assessment
Patients failing the SNLG-modified ACE-27 rating scale (Table 1) were designated “frail” and excluded from the VEPEMB study. No patients in this category achieved CR on any form of therapy used (Table 4). By contrast, 67 “nonfrail” VEPEMB-treated patients entered CR. All 13 frail patients died (12 of progressive HL and 1 of sepsis) with a median OS of 7 months. This is compared with 34 of 103 “nonfrail” VEPEMB-treated patients who died (P < .001). Significance was retained when data from all 157 patients treated with VEPEMB, ABVD, and CLVPP were compared, including the outcome for 7 “frail” patients in that group treated with curative intent (Table 5). This demonstrates the value of objectively identifying a subgroup unsuitable for studies of aggressive multiagent chemotherapy.
Basic clinical and laboratory characteristics of patient populations
| Item . | VEPEMB . | ABVD . | CLVPP . | Other . | P* . |
|---|---|---|---|---|---|
| Total no. of patients | 103 | 35 | 19 | 18 | |
| Sex | .993 | ||||
| Male | 53 (51.5) | 15 (42.9) | 13 (68.4) | 9 (50.0) | |
| Female | 50 (48.5) | 20 (57.1) | 6 (31.6) | 9 (50.0) | |
| Median age, y (range) | 73 (60-85) | 66 (60-73) | 75 (61-86) | 77 (61-88) | .024 |
| Stage | .948 | ||||
| Early-stage (1A/2A) | 31 (30.1) | 13 (37.1) | 5 (26.3) | 4 (22.2) | |
| Advanced-stage (all other) | 72 (69.9) | 22 (62.9) | 14 (73.7) | 14 (77.8) | |
| ECOG performance status | .342 | ||||
| Not known | 4 | 5 | 1 | 0 | |
| > 1 | 28 (28.3) | 3 (10.0) | 10 (55.6) | 9 (50.0) | |
| ≤ 1 | 71 (71.7) | 27 (90.0) | 8 (44.4) | 9 (50.0) | |
| Comorbidity Rating Scale | < .001 | ||||
| Not known | 0 | 7 | 5 | 1 | |
| Not frail | 103 (100.0) | 26 (92.9) | 9 (64.3) | 11 (64.7) | |
| Frail | 0 (0.0) | 2 (7.1) | 5 (35.7) | 6 (35.3) | |
| ADL | .153 | ||||
| Not known | 6 | 4 | 3 | 3 | |
| Score < 3 | 7 (7.2) | 1 (3.2) | 2 (12.5) | 2 (13.3) | |
| Score ≥ 3 | 90 (92.8) | 30 (96.8) | 14 (87.5) | 13 (86.7) | |
| IDL | .068 | ||||
| Not known | 7 | 4 | 3 | 3 | |
| Score < 4 | 11 (11.5) | 3 (9.7) | 4 (25.0) | 6 (40.0) | |
| Score ≥ 4 | 85 (88.5) | 28 (90.3) | 12 (75.0) | 9 (60.0) | |
| Histology | .023 | ||||
| Classic HL, not further classified | 18 (17.5) | 2 (5.7) | 2 (10.5) | 3 (16.7) | |
| Lymphocyte-rich classic HL | 4 (3.9) | 2 (5.7) | 1 (5.3) | 2 (11.1) | |
| Mixed cellularity | 34 (33.0) | 10 (28.6) | 8 (42.1) | 3 (16.7) | |
| Nodular sclerosing | 47 (45.6) | 16 (45.7) | 8 (42.1) | 9 (50.0) | |
| Other | 0 (0.0) | 5 (14.3) | 0 (0.0) | 1 (5.6) | |
| Bulk disease | .888 | ||||
| Not known | 7 | 2 | 2 | 1 | |
| Present | 14 (14.6) | 5 (15.2) | 2 (11.8) | 1 (5.9) | |
| Not present | 82 (85.4) | 28 (84.8) | 15 (88.2) | 16 (94.1) | |
| Marrow involvement | .004 | ||||
| Not known | 28 | 13 | 9 | 9 | |
| Present | 25 (33.3) | 4 (18.2) | 2 (20.0) | 1 (11.1) | |
| Not present | 50 (66.7) | 18 (81.8) | 8 (80.0) | 8 (88.9) | |
| No. of nodal sites | .885 | ||||
| Not known | 15 | 5 | 2 | 2 | |
| < 3 | 40 (45.5) | 18 (60.0) | 5 (29.4) | 7 (43.8) | |
| ≥ 3 | 48 (54.5) | 12 (40.0) | 12 (70.6) | 9 (56.3) | |
| Reed-Sternberg EBV | < .001 | ||||
| No result | 9 | 13 | 9 | 9 | |
| Positive | 51 (54.3) | 15 (68.2) | 6 (60.0) | 7 (77.8) | |
| Negative | 43 (45.7) | 7 (31.8) | 4 (40.0) | 2 (22.2) | |
| Hemoglobin | .789 | ||||
| Normal | 38 (36.9) | 18 (51.4) | 5 (26.3) | 5 (27.8) | |
| Abnormal | 65 (63.1) | 17 (48.6) | 14 (73.7) | 13 (72.2) | |
| Albumin | < .001 | ||||
| Unknown | |||||
| Normal | 58 (56.3) | 25 (71.4) | 11 (57.9) | 9 (50.0) | |
| Abnormal | 41 (39.8) | 5 (14.3) | 8 (42.1) | 8 (44.4) | |
| LDH | .376 | ||||
| Not known | 15 | 11 | 2 | 1 | |
| Normal | 29 (33.0) | 11 (45.8) | 5 (29.4) | 8 (47.1) | |
| Abnormal | 59 (67.0) | 13 (54.2) | 12 (70.6) | 9 (52.9) | |
| Hasenclever score | .041 | ||||
| Not known | 27 | 16 | 7 | 6 | |
| High | 11 (14.5) | 4 (21.1) | 5 (41.7) | 2 (16.7) | |
| Intermediate | 41 (53.9) | 9 (47.4) | 4 (33.3) | 8 (66.7) | |
| Low | 24 (31.6) | 6 (31.6) | 3 (25.0) | 2 (16.7) |
| Item . | VEPEMB . | ABVD . | CLVPP . | Other . | P* . |
|---|---|---|---|---|---|
| Total no. of patients | 103 | 35 | 19 | 18 | |
| Sex | .993 | ||||
| Male | 53 (51.5) | 15 (42.9) | 13 (68.4) | 9 (50.0) | |
| Female | 50 (48.5) | 20 (57.1) | 6 (31.6) | 9 (50.0) | |
| Median age, y (range) | 73 (60-85) | 66 (60-73) | 75 (61-86) | 77 (61-88) | .024 |
| Stage | .948 | ||||
| Early-stage (1A/2A) | 31 (30.1) | 13 (37.1) | 5 (26.3) | 4 (22.2) | |
| Advanced-stage (all other) | 72 (69.9) | 22 (62.9) | 14 (73.7) | 14 (77.8) | |
| ECOG performance status | .342 | ||||
| Not known | 4 | 5 | 1 | 0 | |
| > 1 | 28 (28.3) | 3 (10.0) | 10 (55.6) | 9 (50.0) | |
| ≤ 1 | 71 (71.7) | 27 (90.0) | 8 (44.4) | 9 (50.0) | |
| Comorbidity Rating Scale | < .001 | ||||
| Not known | 0 | 7 | 5 | 1 | |
| Not frail | 103 (100.0) | 26 (92.9) | 9 (64.3) | 11 (64.7) | |
| Frail | 0 (0.0) | 2 (7.1) | 5 (35.7) | 6 (35.3) | |
| ADL | .153 | ||||
| Not known | 6 | 4 | 3 | 3 | |
| Score < 3 | 7 (7.2) | 1 (3.2) | 2 (12.5) | 2 (13.3) | |
| Score ≥ 3 | 90 (92.8) | 30 (96.8) | 14 (87.5) | 13 (86.7) | |
| IDL | .068 | ||||
| Not known | 7 | 4 | 3 | 3 | |
| Score < 4 | 11 (11.5) | 3 (9.7) | 4 (25.0) | 6 (40.0) | |
| Score ≥ 4 | 85 (88.5) | 28 (90.3) | 12 (75.0) | 9 (60.0) | |
| Histology | .023 | ||||
| Classic HL, not further classified | 18 (17.5) | 2 (5.7) | 2 (10.5) | 3 (16.7) | |
| Lymphocyte-rich classic HL | 4 (3.9) | 2 (5.7) | 1 (5.3) | 2 (11.1) | |
| Mixed cellularity | 34 (33.0) | 10 (28.6) | 8 (42.1) | 3 (16.7) | |
| Nodular sclerosing | 47 (45.6) | 16 (45.7) | 8 (42.1) | 9 (50.0) | |
| Other | 0 (0.0) | 5 (14.3) | 0 (0.0) | 1 (5.6) | |
| Bulk disease | .888 | ||||
| Not known | 7 | 2 | 2 | 1 | |
| Present | 14 (14.6) | 5 (15.2) | 2 (11.8) | 1 (5.9) | |
| Not present | 82 (85.4) | 28 (84.8) | 15 (88.2) | 16 (94.1) | |
| Marrow involvement | .004 | ||||
| Not known | 28 | 13 | 9 | 9 | |
| Present | 25 (33.3) | 4 (18.2) | 2 (20.0) | 1 (11.1) | |
| Not present | 50 (66.7) | 18 (81.8) | 8 (80.0) | 8 (88.9) | |
| No. of nodal sites | .885 | ||||
| Not known | 15 | 5 | 2 | 2 | |
| < 3 | 40 (45.5) | 18 (60.0) | 5 (29.4) | 7 (43.8) | |
| ≥ 3 | 48 (54.5) | 12 (40.0) | 12 (70.6) | 9 (56.3) | |
| Reed-Sternberg EBV | < .001 | ||||
| No result | 9 | 13 | 9 | 9 | |
| Positive | 51 (54.3) | 15 (68.2) | 6 (60.0) | 7 (77.8) | |
| Negative | 43 (45.7) | 7 (31.8) | 4 (40.0) | 2 (22.2) | |
| Hemoglobin | .789 | ||||
| Normal | 38 (36.9) | 18 (51.4) | 5 (26.3) | 5 (27.8) | |
| Abnormal | 65 (63.1) | 17 (48.6) | 14 (73.7) | 13 (72.2) | |
| Albumin | < .001 | ||||
| Unknown | |||||
| Normal | 58 (56.3) | 25 (71.4) | 11 (57.9) | 9 (50.0) | |
| Abnormal | 41 (39.8) | 5 (14.3) | 8 (42.1) | 8 (44.4) | |
| LDH | .376 | ||||
| Not known | 15 | 11 | 2 | 1 | |
| Normal | 29 (33.0) | 11 (45.8) | 5 (29.4) | 8 (47.1) | |
| Abnormal | 59 (67.0) | 13 (54.2) | 12 (70.6) | 9 (52.9) | |
| Hasenclever score | .041 | ||||
| Not known | 27 | 16 | 7 | 6 | |
| High | 11 (14.5) | 4 (21.1) | 5 (41.7) | 2 (16.7) | |
| Intermediate | 41 (53.9) | 9 (47.4) | 4 (33.3) | 8 (66.7) | |
| Low | 24 (31.6) | 6 (31.6) | 3 (25.0) | 2 (16.7) |
Values in parentheses are percentages of all after exclusion on “not known.”
LDH indicates lactate dehydrogenase.
P values contrast VEPEMB with ABVD, CLVPP, and Other considered as a combined group, and cases “Not known” are excluded.
Factors associated with achievement of CR in patients treated with curative intent using VEPEMB, ABVD, and CLVPP (157)
| Factor . | No. of patients . | Parameter . | P . |
|---|---|---|---|
| Age | 157 | Comparison of age (60-69 y vs 70-79 y vs > 80 y) | .32 |
| Sex | 157 | Male vs female | .85 |
| Stage | 157 | 1 vs 2 vs 3 vs 4 | .15 |
| B symptoms | 157 | Present vs absent | .89 |
| Histologic type | 152 | MC vs NS vs LRCHL vs unclassified | .27 |
| No. of nodal sites | 117 | 1-2 vs 3-4 vs > 4 | .55 |
| Serum LDH | 129 | Normal vs abnormal | .33 |
| Lymphocyte count | 154 | < 1 vs 1-2 vs > 2 × 109/L | .40 |
| Hemoglobin | 156 | 6-7.9 vs 8-9.9 vs 10-11.9 vs > 12 g/dL | .04 |
| ADL | 144 | Maximum score 6 vs < 6 | .001 |
| Comorbidity score (modified ACE-27) | 155 | Frail vs nonfrail | CR, .01 |
| OS, .001 | |||
| IDL | 143 | Score 0-2 vs 3-5 vs 6 vs 7-8 | .01 |
| ECOG | 145 | 0-1 vs 2-3 | .02 |
| Bulk disease | 145 | Yes vs no | .23 |
| Hasenclever Index | 106 | Low vs intermediate vs high | .06 |
| Serum albumin | 148 | Normal vs abnormal | .02 |
| EBV antigen Reed-Sternberg cells(available on VEPEMB patients only) | 94 | Positive vs negative | .71 |
| Factor . | No. of patients . | Parameter . | P . |
|---|---|---|---|
| Age | 157 | Comparison of age (60-69 y vs 70-79 y vs > 80 y) | .32 |
| Sex | 157 | Male vs female | .85 |
| Stage | 157 | 1 vs 2 vs 3 vs 4 | .15 |
| B symptoms | 157 | Present vs absent | .89 |
| Histologic type | 152 | MC vs NS vs LRCHL vs unclassified | .27 |
| No. of nodal sites | 117 | 1-2 vs 3-4 vs > 4 | .55 |
| Serum LDH | 129 | Normal vs abnormal | .33 |
| Lymphocyte count | 154 | < 1 vs 1-2 vs > 2 × 109/L | .40 |
| Hemoglobin | 156 | 6-7.9 vs 8-9.9 vs 10-11.9 vs > 12 g/dL | .04 |
| ADL | 144 | Maximum score 6 vs < 6 | .001 |
| Comorbidity score (modified ACE-27) | 155 | Frail vs nonfrail | CR, .01 |
| OS, .001 | |||
| IDL | 143 | Score 0-2 vs 3-5 vs 6 vs 7-8 | .01 |
| ECOG | 145 | 0-1 vs 2-3 | .02 |
| Bulk disease | 145 | Yes vs no | .23 |
| Hasenclever Index | 106 | Low vs intermediate vs high | .06 |
| Serum albumin | 148 | Normal vs abnormal | .02 |
| EBV antigen Reed-Sternberg cells(available on VEPEMB patients only) | 94 | Positive vs negative | .71 |
MC indicates mixed cellularity; NS, nodular sclerosis; LRCHL, lymphocyte-rich classic HL; and LDH, lactate dehydrogenase.
Registration patients
Treated with ABVD chemotherapy.
ABVD was the most common treatment selected by investigators who elected not to prescribe VEPEMB. This cohort of patients, with a median age of 66 years, had an overall higher performance status than VEPEMB study patients but did include 2 frail patients. Of the 36 patients treated with ABVD as part of the registration study, 1 patient was found to have composite lymphoma and was excluded from analysis. Of 35 evaluable patients, 13 had early-stage disease and 22 had advanced-stage disease. All early-stage patients received more than or equal to 2 courses of treatment plus radiotherapy (3 patients were on a concurrent study whereby radiotherapy was administered on a randomized basis). Of these early-stage patients, 8 of 13 obtained CR (62%) and 5 PR (38%; Figure 2B). One death occurred early after therapy as a result of bleomycin lung toxicity. There were no septic deaths. A total of 22 advanced-stage patients were treated. Ten of 22 (46%) obtained CR, 4 of 22 (18%) obtained PR, and 4 of 22 (18%) had no response or progressed on treatment. Four of 22 (18%) advanced-stage patients had treatment-related septic deaths and were not evaluable for clinical response assessment. One advanced-stage patient in this group was frail on the comorbidity index and received reduced-dose ABVD. At a median follow-up of 3 years, 18 patients are alive in CR.
Treated with CLVPP chemotherapy.
CLVPP has been commonly used in the United Kingdom as therapy for older patients with HL for many years because of low toxicity and perceived good efficacy. Nineteen registration patients were treated with CLVPP and 18 had more than or equal to 2 courses. Of the cohort receiving CLVPP, 5 were frail on the comorbidity index scale. Five patients had early-stage disease; 2 of 5 obtained CR, and 3 of 5 had NR/PD. Fourteen patients had advanced-stage disease. CR was achieved in only 3 (21%) patients, PR in 2, NR/PD in 9.
Prognostic factor analysis of combined cohorts treated with curative intent: VEPEMB, ABVD, and CLVPP (n = 157)
An analysis of the factors associated with CR rate for all patients treated with VEPEMB, ABVD, and CLVPP was undertaken. The factors used were those used in analysis of the VEPEMB cohort alone. This group was selected as all patients were treated with curative intent and the majority (150 of 157) of patients were “nonfrail.” The results are shown in Table 5. The comorbidity rating scale retains its strong association with outcome as no frail patients treated with ABVD or CLVPP achieved CR. The other factors showing significant association with achievement of CR were the ADL score, IDL, serum albumin, hemoglobin, ECOG score, and Hasenclever IPS. These factors were further assessed in a multivariate Cox regression model for OS in which CR remains the only significant predictor of survival (P < .001) and other factors in the model lose significance. The multivariate Cox regression model for PFS in this group again identifies CR as a significant factor (P < .001) along with age linked to failure of comorbidity assessment (P < .001). All other factors were not significant, including stage and EBV status (Table 5).
Miscellaneous treatments
Eighteen patients were treated with therapy other than VEPEMB, ABVD, or CLVPP, either because they were considered by their physician too frail (6 were frail on the comorbidity assessment) to proceed with a standard chemotherapy or because an alternative curative approach was deemed appropriate (Figure 2B schedules). The range of treatment choices demonstrates the heterogeneity of the patient population and physician choice in an unselected population.
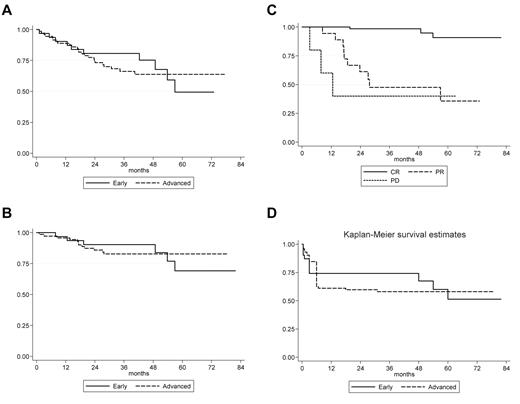
Survival curves—VEPEMB patients. (A) OS of VEPEMB-treated patients by stage of disease at diagnosis. Early-stage patients (n = 31) demonstrate OS at 36 months of 80.7%, and advanced-stage patients (n = 72) demonstrate OS at 36 months of 66.2%. There is no significant difference in OS between the groups (P = .308, log-rank). (B) Disease-specific survival for VEPEMB-treated patients by stage of disease at diagnosis. There is no difference in outcome in OS with HL as cause of death between early-stage (n = 31; 90.3%) and advanced-stage (n = 72; 82.8%) at 36 months (P = .853, log-rank). (C) Disease-specific survival of VEPEMB-treated patients stratified by response to initial therapy. Survival for patients achieving CR (n = 67) on VEPEMB at 36 months was 98.4% and is significantly different (P = .001, log rank) from those with PR (n = 18 with 10 HL deaths) and NR/PD (n = 5 with 3 HL deaths) where survival was 47.5% and 40%, respectively. (D) PFS for VEPEMB-treated patients by stage of disease at presentation. Intention-to-treat PFS for whole VEPEMB cohort (n = 103) for early and advanced stages demonstrates PFS of 74% and 58%, respectively, at 36 months. Thirteen patients were not assessable for response. Eighteen PR patients and 5 NR/PD patients account for early events in these curves.
Survival curves—VEPEMB patients. (A) OS of VEPEMB-treated patients by stage of disease at diagnosis. Early-stage patients (n = 31) demonstrate OS at 36 months of 80.7%, and advanced-stage patients (n = 72) demonstrate OS at 36 months of 66.2%. There is no significant difference in OS between the groups (P = .308, log-rank). (B) Disease-specific survival for VEPEMB-treated patients by stage of disease at diagnosis. There is no difference in outcome in OS with HL as cause of death between early-stage (n = 31; 90.3%) and advanced-stage (n = 72; 82.8%) at 36 months (P = .853, log-rank). (C) Disease-specific survival of VEPEMB-treated patients stratified by response to initial therapy. Survival for patients achieving CR (n = 67) on VEPEMB at 36 months was 98.4% and is significantly different (P = .001, log rank) from those with PR (n = 18 with 10 HL deaths) and NR/PD (n = 5 with 3 HL deaths) where survival was 47.5% and 40%, respectively. (D) PFS for VEPEMB-treated patients by stage of disease at presentation. Intention-to-treat PFS for whole VEPEMB cohort (n = 103) for early and advanced stages demonstrates PFS of 74% and 58%, respectively, at 36 months. Thirteen patients were not assessable for response. Eighteen PR patients and 5 NR/PD patients account for early events in these curves.
Discussion
Both the clinical management of older patients with cancer and the design of research studies pertinent to this population remain major challenges. This is particularly true for HL. In younger patients, HL is cured in the majority, but progress has been very disappointing for older patients, especially those with advanced-stage disease. Population-based studies have shown that approximately 20% of HL occurs in patients older than 60 years.1 Only a small number of clinical studies have been devoted to this subset of HL patients, despite a clear lack of improvement in clinical outcome over the years.2,3 As a result, we have previously suggested that HL in the elderly should be regarded as an “Orphan disease.”10
In the 1990s and early 2000s, a small number of retrospective studies of older HL patients were reported.13,15-17 The common findings were as follows: delivering chemotherapy is difficult in this cohort; complications of chemotherapy, including septic death, are more common than in younger patients; response rates and OS are unsatisfactory and inconsistent between studies, particularly for patients with advanced-stage disease. Such studies did not define a way forward. The SHIELD study approach evaluated the problem in a new way with incorporation of both a phase 2 trial and a registry-based analysis in United Kingdom centers. The study was open to non–United Kingdom centers, and the German GHSG entered 57 patients onto the registration arm of SHIELD. These patients were part of the GHSG-BACOPP phase 2 study. They have not been included in the present discussion as outcome data on this cohort have been published previously.7
The SHIELD study was designed to reevaluate the VEPEMB protocol devised in Italy.9 In the Italian study, VEPEMB was investigated in 105 patients (48 with early-stage disease and 57 with advanced-stage disease). The study included comorbidity assessments and modification of chemotherapy in 18 (17%) with the poorest comorbidity scores. CR rates in the Levis et al study in early-stage disease were excellent at 98% (failure-free survival 79% at 5 years).9 A 58% CR rate in advanced-stage disease (failure-free survival 34% at 5 years) was seen in that study.
The approach adopted in the SHIELD study was designed to use VEPEMB as standard therapy but to use an objective comorbidity rating scale for patient selection.11 This was introduced in an attempt to exclude, in an objective way, “frail” patients from treatment that could be deleterious while allowing physicians to use and register other curative treatment approaches. The result was that the recruitment to the SHIELD VEPEMB study was numerically similar to that of the Italian study; and in addition, 35 patients treated with ABVD were evaluated prospectively. Levis et al demonstrated that the use of VEPEMBx3 plus involved field radiotherapy produces excellent CR rates in patients with early-stage disease,9 and we have confirmed this. CR was achieved in 74% of patients (ITT) with early-stage HL with 3-year OS and PFS of 81% and 74%, respectively. We also confirm that 61% of patients (ITT) with advanced-stage disease obtained CR. Unlike the Italian study, the VEPEMB-induced remissions have been durable. The incidence of treatment-related septic death was 3 of 103 (3%) in our study, but 4 of 103 malignancies occurred during follow-up bringing the treatment-related death rate to 7%.
One VEPEMB-treated patient was thought to have pulmonary fibrosis related to bleomycin toxicity. By contrast, in a cohort of 95 retrospectively reviewed patients reported by Evens et al, in which ABVD was used as primary therapy in 70% of patients, the incidence of pulmonary bleomycin toxicity was 32%.18 A similar level of pulmonary toxicity was also noted by Evens et al when reviewing ABVD in the context of the Stanford V versus ABVD study when applied to elderly patients.19 This finding, taken with the unsatisfactory clinical outcomes reported in the combined Stanford V, ABVD elderly cohort,19 suggests that use of VEPEMB, where the bleomycin dose is only half that of ABVD per 28-day sequence of treatment, might be a safer option.
In the SHIELD study, the treating physicians opted to give ABVD to 35 patients of whom 22 had advanced-stage disease. In age terms, this was a more favorable group than the VEPEMB patients (median age, 66 vs 73 years but did include 2 “frail” patient). The distribution of early-stage and advanced-stage disease stages was similar in the 2 groups (Table 4). The CR rate for ABVD-treated patients was inferior to that with VEPEMB (overall 51% CR rate, 62% in those with early-stage disease, 45% in those with advanced-stage disease). These response rates were lower than expected. A striking feature was treatment-related septic death in 4 of 35 (11%) patients in all and 4 of 22 (18%) of those with advanced-stage disease. These toxicity data are at variance with those of Levis et al20 but are consistent with data from an analysis of ABVD versus Stanford V for patients older than 60 years.19 In this latter study, ABVD was poorly tolerated and resulted in combined CR rate (ABVD/Stanford V combined) of 65% for the older than 60 years group with 3-year and 5-year freedom from progression of 55% and 46%, respectively.
The SNLG modified ACE-27 comorbidity rating system was successfully applied to the VEPEMB study to determine frailty on the basis of comorbidity. None of those excluded by frailty assessments completed or responded to chemotherapy, and it is unlikely that they would have tolerated VEPEMB but, if included on study, would have substantially affected the results. This must be borne in mind when comparing these data to those from other trials, which did not recommend exclusion on the basis of frailty assessments.
Recently, Evens et al in their retrospective assessment of 95 cases have defined a model in which OS in elderly HL patients can be predicted based on age (> 70 years) and loss of ADLs.18 We confirm the finding that ADL assessment is of clinical value along with the assessment of IDL (Table 5). The SNLG-modified ACE-27 demonstrates a difference in survival outcome between all VEPEMB-treated patients and the frail VEPEMB-treated group (P = .001). The SNLG-modified ACE-27 comorbidity rating system appears to provide a robust, objective approach to patient selection for the study of intensive treatments in this age group of patients. If linked to the factors identified by Evens et al this could provide a basis for patient selection in subsequent studies.
It is important to consider the results of the SHIELD program in the context of other international efforts, including 3 trials from the GHSG7,8,21 and 1 from the Italian group.20 The first study from the GHSG used BACOPP. A total of 65 patients 60 to 75 years of age were treated. All were assigned to receive 6 to 8 courses of BACOPP. At a median follow-up of 33 months, 85% obtained CR, 30% had died, and there were 7 treatment-related deaths. These are good results but compromised by 12% treatment-related mortality.7
The GHSG HD9 elderly trial21 randomized patients between COPP/ABVD or BEACOPP baseline. Whereas BEACOPP showed good anti-HL activity with freedom-from-treatment-failure of 74% versus 55%, this did not translate to better 5-year OS (50% for both regimens), mainly because of 21% treatment-related deaths after BEACOPP compared with 8% after COPP/ABVD. These data once again highlight the difficulty of balancing good antitumor effect against potentially life-threatening toxicity in this population.
The most recent data from the GHSG group relate to another phase 2 study of PVAG in 59 patients.8 The median age was 68 years with age of recruitment limited to 60 to 75 years; 93% of patients had advanced-stage disease. The 3-year OS and PFS were 66% and 58%, respectively. The GHSG caution that the population of patients might not be considered representative of HL elderly and suggest more stringent use of geriatric assessments to create a more uniform evaluation of patient populations.22,23
In an abstract outlining their outcome data for 54 patients randomized between ABVD (n = 26) and VEPEMB (n = 28), the Italian Group found: CR rates for ABVD and VEPEMB of 86% and 77%, respectively (not significant). At 3 years, the relapse-free survival was 57% versus 50% (not significant) and OS 79% versus 60% (not significant). No toxic deaths were reported in either arm.20
When comparing these studies, including SHIELD, it is important to consider the selection bias applicable in each case, differences in definition of early- and advanced-stage disease, and the recording/use of comorbidity scoring systems. In summary, the conclusions listed below may assist others in successfully planning and executing future studies in HL in the elderly. Such studies will certainly include combinations of chemotherapy, such as VEPEMB, or alternatives in combination with the very interesting agent SGN35 (Brentuximab Vedotin: an antibody-drug conjugate directed at CD30-expressing cells). This compound has emerged recently as a highly active agent in relapsed or refractory HL in younger patients.24 We would caution against use of SGN35 in the over 60-year-old age group in combination with ABVD given the toxicity and modest efficacy of ABVD reported here and by others in this age group.
1. VEPEMB therapy provides satisfactory disease control in early- and advanced-stage disease with acceptable toxicity and sustained remission in those who have a complete response.
2. ABVD in its standard form has demonstrated substantial toxicity and only moderate efficacy in this age group and should not be automatically considered as standard therapy.18,19
3. VEPEMB has demonstrated minimal pulmonary toxicity in this and previous studies.
4. The SNLG modified ACE-27 comorbidity scale appears to be a robust tool for assessing suitability for multiagent chemotherapy and, if linked to other factors such as ADL, IDL, and ECOG, might facilitate the enrolment of more uniform/comparable patient cohorts into HL elderly studies in future. The International Prognostic Score did not have prognostic significance in the VEPEMB cohort but was of value in the overall cohort (Table 5).
5. The outcome of patients designated frail by comorbidity score is a major concern. Treatment with the newer agent SGN 35 alone in such patients might provide better results than with standard chemotherapy.
6. The on-line program of data collection and comorbidity assessment used in SHIELD has been found to be robust and user friendly. It allowed a rare disease to be studied in detail in multiple cooperating centers at an acceptable cost with small numbers of personnel.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Faculty of Medicine at Newcastle University and the Newcastle on Tyne Hospitals NHS Foundation Trust for research facilities and support.
This work was supported by Bone Marrow and Stem Cell Transplant 2000: The Millenium Fund (registered United Kingdom charity).
Authorship
Contribution: S.J.P. designed the study, wrote protocol, obtained funding, performed research, collected, analyzed, and interpreted data, and was the lead manuscript writer; J.W. codesigned the study, wrote the protocol, performed research, collected and analyzed data, cowrote the manuscript, performed trial management, and organized all ethical and regulatory issues; G.J. performed research, analyzed and interpreted data, and cowrote the manuscript; G.C.W. collected and computed data, assisted with study administration, and cowrote the manuscript; H.H.L. codesigned the study (radiotherapy), performed research, collected data, and cowrote the manuscript; T.M.-F. codesigned the study (laboratory) and protocol, collected data and laboratory samples, and cowrote the manuscript; D.C. performed research, coordinated data collection (Scotland), coanalyzed data, and cowrote the manuscript; M.J.G. performed research and coordinated data collection (Durham); K.M.W. codesigned the study (pathology), performed research, and analyzed data; R.J.Q.M. codesigned the study (statistical input), performed research, analyzed and interpreted data, and cowrote the manuscript; P.W.J. interpreted data, analyzed data (statistics), and cowrote the manuscript; and J.R.G. codesigned the study (pathology review and experimental input), performed research, coordinated histologic review, analyzed data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of participants in the SHIELD study appears in the online supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Stephen J. Proctor, Academic Haematology, Medical School, Newcastle University, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; e-mail: s.j.proctor@newcastle.ac.uk.