Abstract
Mammalian erythroblasts undergo enucleation, a process thought to be similar to cytokinesis. Although an assemblage of actin, non-muscle myosin II, and several other proteins is crucial for proper cytokinesis, the role of non-muscle myosin II in enucleation remains unclear. In this study, we investigated the effect of various cell-division inhibitors on cytokinesis and enucleation. For this purpose, we used human colony-forming unit-erythroid (CFU-E) and mature erythroblasts generated from purified CD34+ cells as target cells for cytokinesis and enucleation assay, respectively. Here we show that the inhibition of myosin by blebbistatin, an inhibitor of non-muscle myosin II ATPase, blocks both cell division and enucleation, which suggests that non-muscle myosin II plays an essential role not only in cytokinesis but also in enucleation. When the function of non-muscle myosin heavy chain (NMHC) IIA or IIB was inhibited by an exogenous expression of myosin rod fragment, myosin IIA or IIB, each rod fragment blocked the proliferation of CFU-E but only the rod fragment for IIB inhibited the enucleation of mature erythroblasts. These data indicate that NMHC IIB among the isoforms is involved in the enucleation of human erythroblasts.
Introduction
During erythropoiesis, stem cells undergo lineage specific commitment and generate erythroid progenitor cells through cellular division events including nuclear (mitosis) and cytoplasmic (cytokinesis) division. These progenitor cells consist of immature and mature erythroid progenitors, the burst-forming unit-erythroid (BFU-E) and the colony-forming unit-erythroid (CFU-E), respectively. The BFU-E can be considered as a progenitor of the CFU-E. Indeed, after 6 to 7 days in culture, cells generated from human BFU-E have all the functional characteristics of CFU-E1. After an additional 6 to 7 days in culture, human CFU-E proliferate and differentiate into mature erythroblasts.1 Terminally differentiated erythroblasts in mammals expel their nuclei via a process termed enucleation, becoming reticulocytes and subsequently mature erythrocytes. The nucleus separates from the remainder of the cell and is phagocytosed by reticular cells such as macrophages (for a review, see Chasis et al2 ).
Enucleation of erythroblasts is thought to occur through a process similar to cytokinesis. Several general principles apply to cytokinesis. Firstly, the microtubule cytoskeleton plays an important role in both the choice and positioning of the division site. Once this site is chosen, the local assembly of the actomyosin contractile ring remodels the plasma membrane. Finally, membrane trafficking to, and membrane fusion at the division site result in the physical separation of the daughter cells, a process termed abscission (for reviews, see Barr et al3 and Glotzer et al4 ). Although modulation of the actomyosin cytoskeleton is crucial for proper cytokinesis, there is a paucity of information regarding how non-muscle myosin II contributes to enucleation.
Several investigations have studied the molecular mechanisms underlying the enucleation of mammalian erythroblasts. Koury et al used murine splenic erythroblasts infected with the anemia-inducing strain of Friend-virus (FVA cells), and demonstrated that filamentous actin (F-actin) accumulated in the contractile ring.5 They also showed that the treatment of FVA cells with cytochalasin D blocked nuclear extrusion, while the addition of colchicine, vinblastine or taxol did not affect enucleation.5 Based on these findings, they concluded that F-actin plays an important role in enucleation, while microtubules do not. It has also been shown that Rac 1 GTPases and their downstream effector mDia2 play important roles in the cytoskeletal reorganization that leads to the extrusion of the pycnotic nucleus from late-stage erythroblasts.6 Recently, important roles for Myc,7 Claudin 138 (a member of the Claudin family of tight junction proteins), histone deacetylase 2,9 and membrane trafficking10 have been reported in the regulation of terminal maturation in mammalian erythroid cells.
Non-muscle myosin II is a major cytoskeletal protein that interacts with actin to contribute to cellular processes such as cell migration,11 cell adhesion,12 and cytokinesis.13 In mammals there are 3 non-muscle myosin II isoforms, each composed of one pair of heavy chains and 2 pairs of light chains. Three separate genes (Myh 9, Myh10, and Myh 14) encode the non-muscle myosin heavy chains (NMHCs; NMHC IIA, IIB, and IIC) in chromosomes 22q11.2, 17p13, and 19q13, respectively.14-16 These isoforms share considerable homology and some overlapping functions, yet they exhibit differences in enzymatic properties, subcellular localization, molecular interaction and tissue distribution (for a review, see Even-Ram et al17 ). The mechanistic knowledge about non-muscle myosin II isoforms in enucleation may help us to explore the unknown NMHC abnormalities in hematologic disorders. Indeed, deficiency of non-muscle myosin II isoform can cause human disease. The 4 main autosomal dominant disorders are related to mutations in Myh 9, the gene for the NMHC IIA: May-Hegglin, Fechtner, Sebastian and Epstein (for a review, see Kunishima et al18 ). The common feature in all 4 syndromes is macrothrombocytopenia, and granulocyte inclusion bodies characterize the first 3 syndromes. Some patients later show onset of deafness, cataracts, and glomerulonephritis.18
In this study, we investigated the role of myosin in human erythroblast enucleation in association with other cytoskeletal molecules. The efficacy of inhibitors for cell division often varies depending on the species of the cell observed and their redundancy in the cells themselves.19 Therefore, efficient inhibitors of cell division in human primary erythroid cells were selected using human CFU-E generated from purified CD34+ cells to clarify the possible efficacy of these inhibitors on the enucleation of erythroblasts. Here we show that the inhibition of non-muscle myosin II ATPase by blebbistatin completely blocks enucleation of human erythroblasts. When the function of NMHC IIA or IIB was inhibited by an exogenous expression of myosin rod fragment, both rod fragments blocked the proliferation of CFU-E and the rod fragment for IIB inhibited the enucleation of mature erythroblasts. These data indicate that the enucleation of human erythroblasts involves non-muscle myosin IIB.
Methods
Reagents and antibodies
BSA, IMDM and propidium iodide (PI) were purchased from Sigma-Aldrich. FCS, penicillin and streptomycin were obtained from Flow Laboratories Inc. Insulin (porcine sodium, activity 28.9 U/mg) was obtained from Wako Pure Chemical Industries. IL-3 and SCF were kind gifts from Kirin Brewery Co Ltd, and erythropoietin (EPO) and G-CSF were from Chugai Pharmaceutical Co. Vitamin B12 was purchased from Eisai Co Ltd and folic acid was from Takeda Pharmaceutical Co Ltd. Triton X-100 was obtained from Wako Pure Chemical Industries. Diaminobenzidine-substrate chromogen and fuchsin-substrate chromogen systems were from Dako. RNase (Type III-A) was from Sigma-Aldrich. MACS MicroBeads for Indirect Magnetic Labeling was from Miltenyi Biotec. FITC-labeled and phycoerythrin (PE)–labeled antibodies for glycophorin A (GPA; JC159) were from Dako. Alexa Fluor 488– or Alexa Fluor 546–conjugated goat IgG directed against rabbit and mouse IgG were from Molecular Probes. Fc-blocking antibody (anti-CD16/32, clone: 93) was from eBioscience. Normal mouse and goat sera and rabbit immunoglobulins were from CellSignaling Technology. Alexa Fluor 488 phalloidin was from Invitrogen. Rabbit polyclonal antibody to non-muscle myosin IIA was from Sigma-Aldrich or Covance. Rabbit polyclonal antibody to non-muscle myosin IIB was from Abcam or Cell Signaling Technology. Mouse monoclonal antibody to tublin-α was from Neo Markers. Goat anti–mouse IgG-HRP was from Santa Cruz Biotechnology Inc. Anti–rabbit IgG, HRP-linked antibody and myosin light chain 2 antibody sampler kit were from Cell Signaling Technology. Amersham ECL Plus Western Blotting detection reagents were from GE Healthcare. Novex Sharp protein standard and NuPAGE Novex Bis-Tris mini gels were from Invitrogen.
Cell division inhibitors
Blebbistatin, a small molecule inhibitor that shows a high affinity and selectivity toward non-muscle myosin II ATPase,20 and NSC23766, a specific inhibitor of Rac1 GTPases21 that is known to inhibit enucleation,6 were purchased from Calbiochem. Cytochalasin D, an inhibitor of actin polymerization that is known to inhibit enucleation,5 and colchicine, an inhibitor of microtubules that are known to not be directly involved in enucleation5 (for a review, see Wilson et al22 ), were purchased from Sigma-Aldrich. Y27632, a well-established inhibitor of ROCK that is a regulator of myosin phosphorylation,23,24 was purchased from Enzo Life Sciences, Inc. Monastrol, an inhibitor of kinesin Eg5 which is a microtubule-based motor that plays a critical role in mitosis as it mediates centrosome separation and bipolar spindle assembly and maintenance,25 was from Merck.
Cell preparations
G-CSF mobilized human peripheral blood CD34+ cells were purified from healthy volunteers as described previously,26 and stored in liquid nitrogen until required. Informed consent was obtained from each subject before their entry into this study, and the study was pre-approved by the Akita University Graduate School of Medicine Committee for the Protection of Human Subjects.
For the generation of erythroid progenitor cells, CD34+ cells were thawed and prepared for culture as previously described.27 Cells were cultured in IMDM erythroid medium containing 20% FBS, 10% heat-inactivated pooled human AB serum, 1% BSA, 10 μg/mL insulin, 0.5 μg/mL vitamin B12, 15 μg/mL folic acid, 50nM β-mercaptoethanol (β-ME), 50 U/mL penicillin and 50 μg/mL streptomycin in the presence of 50 ng/mL IL-3, 50 ng/mL SCF and 2 IU/mL EPO. Cells were maintained at 37°C in a 5% CO2 incubator as described previously.27 After 7 days in culture, cells (day 7 cells, D7) were harvested and washed 3 times with IMDM containing 0.1% BSA and stored at 4°C until required. The maturation levels of the day 7 cells were found to be similar to that of the colony-forming unit-erythroid (CFU-E), a finding that has been reported elsewhere.27,28 As a result, day 7 cells are described as CFU-E throughout this report.
Aliquots of CFU-E were also cultured in the erythroid medium with EPO alone, but without β-ME, SCF and IL-3, and then cultured for additional days to induce differentiation with or without various inhibitors of cell division. The volume of inhibitors, H2O, PBS and DMSO vehicle solutions was fixed to 5% of the erythroid medium. The final concentration of DMSO in the erythroid medium was fixed to 0.2% (vol/vol) as DMSO over this concentration is known to be toxic for human erythroid progenitors. The cells were harvested at various time points, washed 3 times with IMDM containing 0.1% BSA, resuspended in IMDM containing 0.1% BSA and stored at 4°C until required.
For the evaluation of the enucleation ratio, the cells were spun onto slides using a Cytospin 3 (Shandon Lipshaw Inc) and stained with May-Grünwald-Giemsa or o-dianisidine (for hemoglobin staining) and hematoxylin. Enucleation was defined as the expulsion of the nucleus to the outside of the reticulocyte. Reticulocytes that are touching expulsed nuclei or that have thin, connecting strand of cellular material between the reticulocyte and the nucleus were also considered the earliest cell that has enucleated (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The enucleation ratio of these cytospun cells was similar to that of cells prepared without mechanical force.1 The enucleation ratio was calculated as [Enucleation ratio = erythrocytes/(erythrocytes+erythroblasts)] × 100% and by counting 300 cells including erythrocytes and erythroblasts on each slide. Triplicate cultures were used at each time point. The yield and viability were measured by dye exclusion using 0.2% trypan blue dye and a hemocytometer.
Cell cycle distribution
Cells were harvested, washed with cold PBS and fixed in 70% ethanol. The cells were then stored at −20°C until analysis. The fixed cells were centrifuged at 200g, washed with cold PBS twice, and RNase A was added at a final concentration of 0.5 mg/mL. The cells were then incubated for 10 minutes at 37°C. Next, 25 μg/mL PI was added and the cells were incubated for 30 minutes at room temperature in the dark. The cells were analyzed using a FACS Calibur instrument (BD Biosciences) equipped with CellQuest 3.3 software as reported previously.29 Multicycle Version 4.0.0.4 cell cycle analysis software (Beckman Coulter) was used to determine the percentages of cells in the different cell cycle phases.
Confocal microscopy
Fluorescence staining was imaged using a Confocal Laser Scanning Microscope 510 (LSM510; Carl Zeiss Microscope Systems) equipped with a 100× objective lens and a 10× camera lens (Carl Zeiss Microscope Systems) at zoom 3, as reported elsewhere.29 Fluorochromes were excited using an argon laser at 488 nm for Alexa 488. Detector slits were configured to minimize cross talk between channels and processed using a software package (LSM510 Version 3.2) and Adobe Photoshop (Adobe Systems).
Construction of plasmid DNA
The myosin II tailpiece determines its paracrystal structure, filament assembly properties, and cellular localization.30 To study the role of non-muscle myosin II isoforms in enucleation of human erythroblasts by the exogenous expression of their rod fragments, we constructed the plasmids encoding the rod fragments of the 2 NMHC-II isoforms as N-terminal GFP-fused protein. DNA fragments encoding Leu 1666 - Glu 1961 of NMHC IIA (ARF296) and Phe 1672–Glu 1976 of NMHC IIB (BRF305) were amplified by PCR as described elsewhere.31,32 Each of them was subcloned into the HindIII-BamH1 sites of pEGFP-C3 (Clontech) to generate the pEGFP-ARF296 and pEGFP-BRF305, respectively.
Transfection
Transfected cells were obtained with the Amaxa nucleofection method. The Amaxa Nucleofector system (Lonza Cologne AG) was used as described by the manufacturer. Briefly, a pellet of 2-5 × 106 cells in 100 μL of Amaxa Human CD34+ Cell Nucleofector solution wasmixed with 5 μg of pEGFP plasmid and subjected to nucleofection with a specific predefined program (Program U-008). After 24 hours of incubation, the cells were harvested and washed twice with IMDM containing 0.3% BSA. The dead cells were removed using an Annexin V MicroBead Kit (Miltenyi Biotec) and then incubated in the erythroid medium. GFP-positive cells were collected using a cell sorter (Dako Cytomation MoFlo).
Western blot analysis
CD34+ cells were incubated in erythroid medium for the indicated days (day 7, 10-12 cells). Western blot analysis was carried out according to the manufacturer's protocol (Invitrogen).
Statistical analysis
Statistical analysis was performed using the Student t test for parametric data and the Mann-Whitney test for nonparametric data. A 2-tailed P value < .05 was accepted as statistically significant.
Results
Myosin inhibitors block cell division of human CFU-E
In this study, efficient inhibitors of cell division in human primary erythroid cells were selected using human CFU-E generated from purified CD34+ cells (Figures 1–2). As cellular division consists of both mitotic and cytokinetic events, and given that the inhibition of either step should block cell proliferation, efficient inhibitors were defined as those that blocked the proliferation of CFU-E.
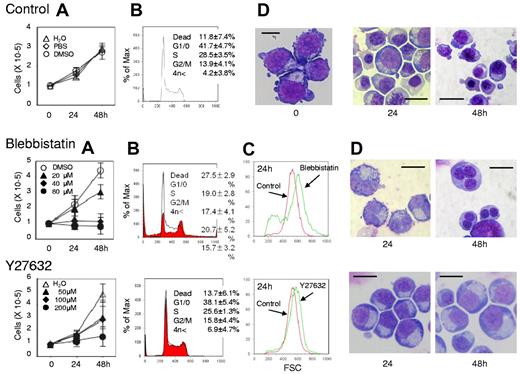
Myosin inhibitors block cell division of human CFU-E. Human CFU-E generated from purified CD34+ cells were cultured for the indicated periods in the presence of EPO with or without various concentrations of inhibitors for non-muscle myosin II ATPase (blebbistatin) and a myosin activator Rho kinase (Y27632). (A) Effects of inhibitors and vehicle on the proliferation of CFU-E. Results are presented as the mean ± SD of 3 independent experiments. (B) Cell cycle analysis of cells cultured for 24 hours with (red areas) or without (solid lines) 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown and is presented as the mean ± SD. (C) Size distribution analysis of CFU-E before (0 hours) and after 24 hours culture (red) with 80μM blebbistatin (green, middle panel) and 200μM Y27632 (green, bottom panel). A representative result of 3 independent experiments is shown. (D) May-Grünwald-Giemsa staining of cells cultured for 24 and 48 hours with or without 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
Myosin inhibitors block cell division of human CFU-E. Human CFU-E generated from purified CD34+ cells were cultured for the indicated periods in the presence of EPO with or without various concentrations of inhibitors for non-muscle myosin II ATPase (blebbistatin) and a myosin activator Rho kinase (Y27632). (A) Effects of inhibitors and vehicle on the proliferation of CFU-E. Results are presented as the mean ± SD of 3 independent experiments. (B) Cell cycle analysis of cells cultured for 24 hours with (red areas) or without (solid lines) 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown and is presented as the mean ± SD. (C) Size distribution analysis of CFU-E before (0 hours) and after 24 hours culture (red) with 80μM blebbistatin (green, middle panel) and 200μM Y27632 (green, bottom panel). A representative result of 3 independent experiments is shown. (D) May-Grünwald-Giemsa staining of cells cultured for 24 and 48 hours with or without 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
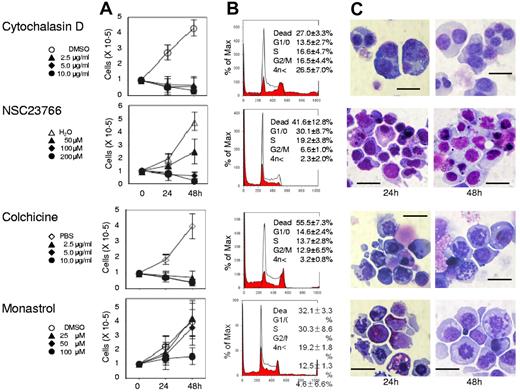
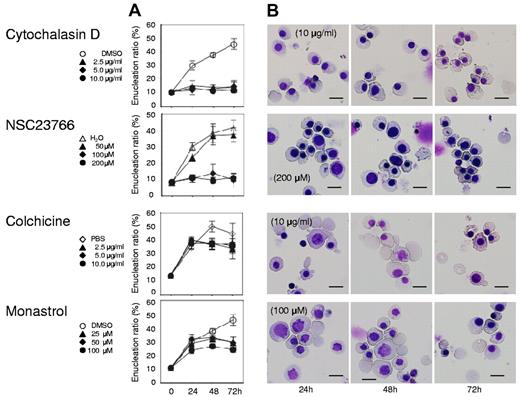
Actin, tubulin, and Eg5 inhibitors block cell division of human CFU-E. Human CFU-E were cultured for the indicated periods in the presence of EPO with or without various concentrations of inhibitors for actin polymerization (cytochalasin D and NSC23766), tubulin (colchicine) and Eg5 (monastrol). (A) Effects of inhibitors and vehicle on the proliferation of CFU-E. Results presented are the mean ± SD of 3 independent experiments. (B) Cell cycle analysis of cells cultured for 24 hours with (red areas) or without (solid lines) 10μg/mL cytochalasin D, 200μM NSC23766, 10 μg/mL colchicine and 100μM monastrol. A representative result of 3 independent experiments is shown and is presented as the mean ± SD (C) May-Grünwald-Giemsa staining of cells cultured for 24 and 48 hours with or without 10 μg/mL cytochalasin D, 200μM NSC23766, 10 μg/mL colchicine and 100μM monastrol. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
Actin, tubulin, and Eg5 inhibitors block cell division of human CFU-E. Human CFU-E were cultured for the indicated periods in the presence of EPO with or without various concentrations of inhibitors for actin polymerization (cytochalasin D and NSC23766), tubulin (colchicine) and Eg5 (monastrol). (A) Effects of inhibitors and vehicle on the proliferation of CFU-E. Results presented are the mean ± SD of 3 independent experiments. (B) Cell cycle analysis of cells cultured for 24 hours with (red areas) or without (solid lines) 10μg/mL cytochalasin D, 200μM NSC23766, 10 μg/mL colchicine and 100μM monastrol. A representative result of 3 independent experiments is shown and is presented as the mean ± SD (C) May-Grünwald-Giemsa staining of cells cultured for 24 and 48 hours with or without 10 μg/mL cytochalasin D, 200μM NSC23766, 10 μg/mL colchicine and 100μM monastrol. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
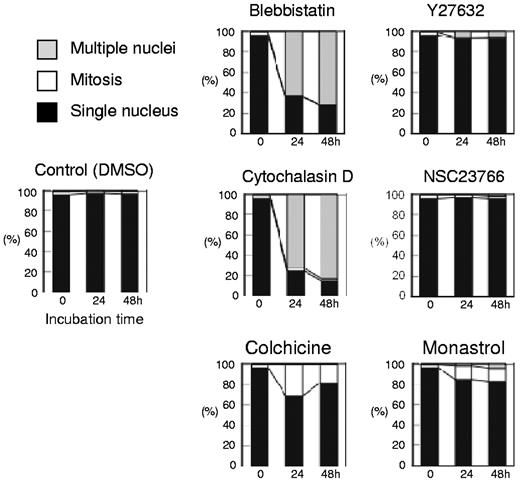
Figure 1A presents a time course analysis of the total cell number when human CFU-E were cultured with or without various concentrations of blebbistatin and Y27632. Blebbistatin and Y27632 were found to inhibit CFU-E proliferation in a dose-dependent manner. Cell cycle analysis demonstrated that blebbistatin decreased the number of cells in the G1/G0/S phase and resulted in the accumulation of cells in the G2/M phase, a finding that was accompanied by an increase in dead cells (Figure 1B). In addition, an increase in cells with high DNA content (Figure 1B) and an increase in larger erythroid progenitors compared with control cells (Figure 1C) were observed, suggesting the presence of multinucleated cells. This finding was confirmed by morphologic analysis (Figures 1D and 3). These data indicate that non-muscle myosin II ATPase is essential for the cellular division of CFU-E.
Morphologic analysis of cells cultured with or without inhibitors. Differential counts of cells with a single nucleus (closed bars), multiple nuclei (shaded bars), and cells in mitosis (open bars) are shown. Results presented are the mean of 3 independent experiments.
Morphologic analysis of cells cultured with or without inhibitors. Differential counts of cells with a single nucleus (closed bars), multiple nuclei (shaded bars), and cells in mitosis (open bars) are shown. Results presented are the mean of 3 independent experiments.
In contrast, Y27632 slightly increased the number of cells in the G2/M phase, generated larger cells compared with the control cells (Figure 1C) and produced multinucleated cells from 0.7 ± 1.2% to 5.7 ± 1.2% during 48 hours of incubation (n = 3, P < .01; Figures 1D and 3), albeit to a lesser extent than that observed in the presence of blebbistatin (from 0.7 ± 1.2% to 71.0 ± 6.1%; n = 3, P < .01; Figure 3). Western blot analysis showed that Y27632 does not affect the myosin light chain 2 (MLC2) expression, a downstream molecule of ROCK (a regulator of myosin phosphorylation23,24 ), but reduces the phosphorylation of MLC2 at Thr18/Ser19 in CFU-E, with a statistical significance (supplemental Figure 2). Given that the blocking of non-muscle myosin II ATPase and ROCK showed complete inhibition of the proliferative capacity of CFU-E, non-muscle myosin II is suggested to be involved in cell division of human erythroid progenitor cells.
Actin, tubulin, and Eg5 inhibitors block cell division of human CFU-E
Although Koury et al have clearly shown that F-actin plays an important role in enucleation in murine FVA cells while microtubules do not,5 the efficacy of inhibitors for cell division often depends on the species of the cell observed and their redundancy in the cells themselves.19 We therefore reevaluated the efficacy of actin and tubulin/kinesin inhibitors on human CFU-E and erythroblasts. As illustrated in Figure 2, cytochalasin D, an inhibitor of actin polymerization, NSC23766, a specific inhibitor of Rac1 GTPases21 that regulate actin polymerization, colchicine, an inhibitor of microtubules,22 and monastrol, an inhibitor of kinesin Eg5, completely blocked CFU-E proliferation. Cytochalasin D decreased the number of cells in the G1/G0/S phase resulting in the accumulation of cells in the G2/M phase (Figure 2B), and produced multinucleated cells (Figures 2C and 3). Interestingly, NSC23766 dramatically increased the total number of dead cells and decreased the number of cells in the mitotic fraction (Figure 2B), and did not produce multinucleated cells (Figures 2C and 3). These data indicate that cytochalasin D and NSC23766 block the cell division of human CFU-E.
Colchicine and monastrol decreased the number of cells in the G1/G0/S phase, while cells accumulated in the G2/M phase, a finding that was accompanied by an increase in the number of dead cells (Figure 2B). Morphologic analysis showed that these inhibitors increased the number of cells undergoing mitosis (Figures 2C and 3). These data indicate that colchicine and monastrol block mitosis, and are efficient inhibitors of cell division in human CFU-E.
Myosin inhibitors block enucleation of human erythroblasts
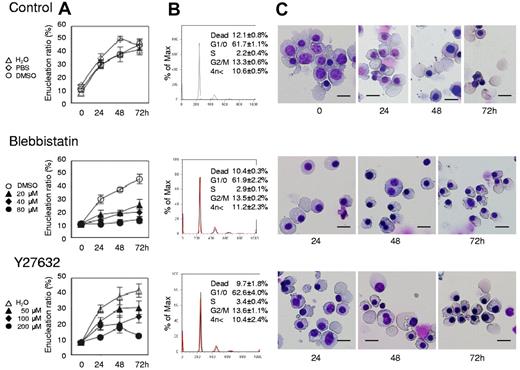
Figure 4A presents a time course analysis of the enucleation ratio when day 11 human mature erythroblasts were cultured with or without various concentrations of blebbistatin and Y27632. During the 72-hour culture period, mature erythroblasts differentiated and started the enucleation process (Figure 4A and C top panel). After beginning the incubation, enucleation was rapidly initiated within 24 hours and continued until 72 hours. Both blebbistatin and Y27632 caused an immediate and significant inhibition of enucleation, in a dose dependent manner (Figure 4A and C middle and bottom panels). Western blot analysis showed that Y27632 reduces the phosphorylation of MLC2 at Thr18/Ser19 in these cells, with a statistical significance (supplemental Figure 2). In contrast to the significant effect on CFU-E (Figure 1B), neither blebbistatin nor Y27632 affects the cell cycle of day 11 mature erythroblasts (Figure 4B). These inhibitors did not affect the level of hemoglobinization of day 11 cells during 72 hours incubation (supplemental Figure 3). These results indicate that most of day 11 erythroblasts consist of mature erythroblasts just before the stage of enucleation and that non-muscle myosin II is essential for the enucleation of human erythroblasts.
Myosin inhibitors block enucleation of human erythroblasts. Human CFU-E were cultured with EPO for an additional 4 days (day 11 cells) and differentiated to the level of mature erythroblasts incipient of enucleation. Mature erythroblasts were then cultured in the presence of EPO with or without various concentrations of inhibitors for non-muscle myosin II ATPase (blebbistatin) and a myosin activator Rho kinase (Y27632). (A) Effects of inhibitors and vehicle on the enucleation of day 11 mature erythroblasts. Results are presented as the mean ± SD of 3 independent experiments. (B) Cell cycle analysis of cells cultured for 24 hours with (red lines) or without (black lines) 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown and is presented as the mean ± SD. (C) May-Grünwald-Giemsa staining of day 11 cells cultured for 24, 48 and 72 hours with or without 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
Myosin inhibitors block enucleation of human erythroblasts. Human CFU-E were cultured with EPO for an additional 4 days (day 11 cells) and differentiated to the level of mature erythroblasts incipient of enucleation. Mature erythroblasts were then cultured in the presence of EPO with or without various concentrations of inhibitors for non-muscle myosin II ATPase (blebbistatin) and a myosin activator Rho kinase (Y27632). (A) Effects of inhibitors and vehicle on the enucleation of day 11 mature erythroblasts. Results are presented as the mean ± SD of 3 independent experiments. (B) Cell cycle analysis of cells cultured for 24 hours with (red lines) or without (black lines) 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown and is presented as the mean ± SD. (C) May-Grünwald-Giemsa staining of day 11 cells cultured for 24, 48 and 72 hours with or without 80μM blebbistatin and 200μM Y27632. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
Actin inhibitors, but not tubulin/Eg5 inhibitors, block enucleation of human erythroblasts
Both cytochalasin D and NSC23766, which are known to inhibit enucleation,5,6 caused an immediate and complete inhibition of enucleation of day 11 human mature erythroblasts (Figure 5 top 2 panels), indicating that actin polymerization is essential for the enucleation of human erythroblasts. In contrast, colchicine, an inhibitor of microtubules that are known to not be directly involved in enucleation,5 did not block enucleation (Figure 5 bottom 3rd panel), indicating that day 11 human mature erythroblasts consist of postmitotic cells just before the stage of enucleation. In addition to the current confirmation of previous reports, we found for the first time that monastrol, an inhibitor of kinesin Eg5 which is a microtubule-based motor that plays a critical role in mitosis,25 does not block enucleation of human erythroblasts (Figure 5 bottom panel).
Actin inhibitors, but not tubulin/Eg5 inhibitors, block enucleation of human erythroblasts. Mature erythroblasts were cultured in the presence of EPO with or without various concentrations of inhibitors for actin polymerization (cytochalasin D and NSC23766), tubulin (colchicine) and Eg5 (monastrol). (A) Effects of inhibitors and vehicle on the enucleation of mature erythroblasts. Results are presented as the mean ± SD of 3 independent experiments. (B) May-Grünwald-Giemsa staining of cells cultured for 24, 48 and 72 hours with or without 10 μg/mL cytochalasin D, 200μM NSC23766, 10 μg/mL colchicine and 100μM monastrol. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
Actin inhibitors, but not tubulin/Eg5 inhibitors, block enucleation of human erythroblasts. Mature erythroblasts were cultured in the presence of EPO with or without various concentrations of inhibitors for actin polymerization (cytochalasin D and NSC23766), tubulin (colchicine) and Eg5 (monastrol). (A) Effects of inhibitors and vehicle on the enucleation of mature erythroblasts. Results are presented as the mean ± SD of 3 independent experiments. (B) May-Grünwald-Giemsa staining of cells cultured for 24, 48 and 72 hours with or without 10 μg/mL cytochalasin D, 200μM NSC23766, 10 μg/mL colchicine and 100μM monastrol. A representative result of 3 independent experiments is shown. Scale bar = 10 μm.
Human erythroblasts express non-muscle myosin IIA and IIB
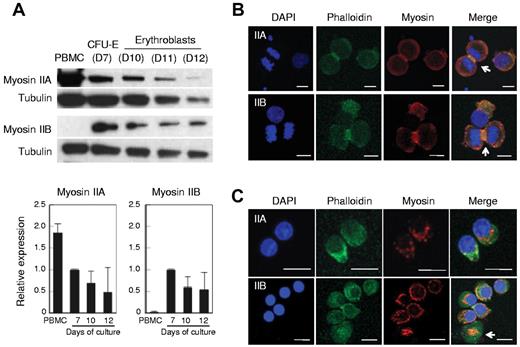
Mammalian cells have 3 isoforms of NMHC, termed IIA, IIB and IIC.15,33,34 Western blot analysis showed that human CFU-E possess both NMHC IIA and IIB. The relative expression levels of NMHC IIA and IIB appeared to decrease as they matured, but the decreases were not statistically significant (Figure 6A). NMHC IIB was extremely low or undetectable in human PBMCs and NMHC IIC was not detected in either human erythroblasts or PBMC (data not shown). The localizations of NMHC IIA and IIB were similar in CFU-E (Figure 6B), although IIB appeared to be more prominent in the contractile ring and colocalized with actin (Figure 6B arrow). In enucleating erythroblasts, NMHC IIA and IIB appeared to localize between the expelling nuclei and the reticulocytes (Figure 6C). Interestingly, NMHC IIB was present on the enucleated reticulocytes in the shape of a small ring that was colocalized with actin, which might indicate the expulsion route of the nuclei expelled from erythroblasts (Figure 6C arrow).
Human erythroblasts express non-muscle myosin IIA and IIB. (A) Western blotting of human PBMCs, CFU-E (day 7 cells, D7) and erythroblasts (D10-D12). D7-D12 indicates days of culture of purified human CD34+ cells to induce erythroid differentiation. At indicated days, the cells were harvested and the protein obtained from 1 × 105 cells was applied to each lane. The relative expression levels of myosin II were normalized with tubulin expression and are the mean ± SD of 3 independent experiments. (B-C) Confocal microscopy of CFU-E (B) and day 12 enucleating mature erythroblasts (C) stained by DAPI, phalloidin and myosin IIA or IIB. NMHC IIA and IIB colocalized with actin (B arrows). In enucleating erythroblasts, NMHC IIB was present on the enucleated reticulocytes in the shape of a small ring colocalized with actin (C arrow).
Human erythroblasts express non-muscle myosin IIA and IIB. (A) Western blotting of human PBMCs, CFU-E (day 7 cells, D7) and erythroblasts (D10-D12). D7-D12 indicates days of culture of purified human CD34+ cells to induce erythroid differentiation. At indicated days, the cells were harvested and the protein obtained from 1 × 105 cells was applied to each lane. The relative expression levels of myosin II were normalized with tubulin expression and are the mean ± SD of 3 independent experiments. (B-C) Confocal microscopy of CFU-E (B) and day 12 enucleating mature erythroblasts (C) stained by DAPI, phalloidin and myosin IIA or IIB. NMHC IIA and IIB colocalized with actin (B arrows). In enucleating erythroblasts, NMHC IIB was present on the enucleated reticulocytes in the shape of a small ring colocalized with actin (C arrow).
Enucleation of human erythroblasts involves non-muscle myosin IIB
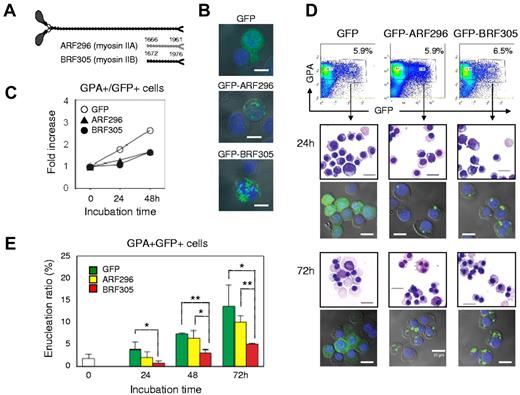
Figure 7A illustrates the rod fragments, ARF296 and BRF305, which are encoded by Leu 1666–Glu 1961 of NMHC IIA and Phe 1672–Glu 1976 of NMHC IIB, respectively.31,32 When ARF296 or BRF305 was exogenously expressed to CFU-E, these rods localized in cytoplasm (Figure 7B) and blocked the proliferation of CFU-E (Figure 7C). To investigate the function of myosin IIA and IIB in the enucleation of erythroblasts, mature erythroblasts (day 10 cells) were transfected with ARF296 and BRF305. The transfected cells were sorted by flowcytometry and cultured for an additional 72 hours (Figure 7D). Confocal microscopy showed that transfected cells maintain GFP-expression during the culture period (Figure 7D). As illustrated in Figure 7E, the enucleation of mature erythroblasts was inhibited by the exogenous expression of BRF305, with statistical significance, while only a slight inhibitory effect of ARF296 was apparent. These data indicate that enucleation of human erythroblasts involves non-muscle myosin IIB.
Enucleation of human erythroblasts involves non-muscle myosin IIB. (A) A schematic presentation of rod fragments: ARF296 (for myosin IIA) and BRF305 (for myosin IIB). (B) Confocal microscopy of CFU-E transfected by pEGFP-ARF296 and pEGFP-BRF305. Scale bar = 10 μm. (C) CFU-E were transfected with pEGFP-ARF296 and pEGFP-BRF305. After 24 hours of incubation, the dead cells were removed and then incubated in the erythroid medium. After culture for the indicated period, the cells were harvested, counted and GPA+/GFP+ cells were analyzed by flowcytometry. Representative data for 2 independent experiments are shown. (D) Mature day 10 erythroblasts were transfected with pEGFP-ARF296 and pEGFP-BRF305. GPA+/GFP+ cells were sorted and cultured in the erythroid medium. After culture for the indicated period, the cells were harvested and stained with May-Grünwald-Giemsa. An aliquot of the cells were observed by confocal microscopy. Scale bar = 10 μm. (E) GPA+/GFP+ cells were cultured in the erythroid medium. At the indicated period, the cells were harvested and counted. Results are presented as the mean ± SD of 3 independent experiments. *P < .05 and **P < .01.
Enucleation of human erythroblasts involves non-muscle myosin IIB. (A) A schematic presentation of rod fragments: ARF296 (for myosin IIA) and BRF305 (for myosin IIB). (B) Confocal microscopy of CFU-E transfected by pEGFP-ARF296 and pEGFP-BRF305. Scale bar = 10 μm. (C) CFU-E were transfected with pEGFP-ARF296 and pEGFP-BRF305. After 24 hours of incubation, the dead cells were removed and then incubated in the erythroid medium. After culture for the indicated period, the cells were harvested, counted and GPA+/GFP+ cells were analyzed by flowcytometry. Representative data for 2 independent experiments are shown. (D) Mature day 10 erythroblasts were transfected with pEGFP-ARF296 and pEGFP-BRF305. GPA+/GFP+ cells were sorted and cultured in the erythroid medium. After culture for the indicated period, the cells were harvested and stained with May-Grünwald-Giemsa. An aliquot of the cells were observed by confocal microscopy. Scale bar = 10 μm. (E) GPA+/GFP+ cells were cultured in the erythroid medium. At the indicated period, the cells were harvested and counted. Results are presented as the mean ± SD of 3 independent experiments. *P < .05 and **P < .01.
Discussion
In this study, we demonstrate for the first time that blebbistatin and Y27632 inhibit the enuclation of human erythroblasts. Blebbistatin is a small molecule inhibitor that shows a high affinity and selectivity toward non-muscle myosin II ATPase,20 while Y27632 is well-established inhibitor of ROCK.23,24 Collectively, these data suggest that the enucleation of human erythroblasts involves non-muscle myosin II. This is of particular interest given that the precise role of myosin in enucleation has remained unclear, even though the formation of the contractile actin ring (CAR) and furrow formation are known to occur on the plasma membrane of enucleating erythroblasts.5,6 As non-muscle myosin II is one of the central mechanisms involved in cytokinesis and is required for furrow formation,22,24,35 our study also suggests that myosin shares a similar mechanistic function between cytokinesis and the enucleation of human erythroblasts.
Although we confirmed that Y27632 inhibits the phosphorylation of MLC2 at Thr18/Ser19, several lines of evidence indicate that activation of myosin II by ROCK is redundant with the action of other kinases such as citron kinase and that Y27632 blocks neither the initiation nor completion of cytokinesis of Hela cells, although it slows cleavage contraction.36,37 Reportedly, ROCK-dependent phosphorylation of the mDia2 is an important determinant of mDia2 activity and this signaling mechanism affects actin polymerization.38 Although further studies are needed, considering that Y27632 produces a limited number of multinucleated cells in the culture of CFU-E, the main mechanism of Y27632 in inhibiting cell division and enucleation may involve the Rac 1 GTPases and their downstream effector mDia2.
In addition to the previous report showing that human erythroblasts express both NMHC IIA and IIB,39 our study demonstrates that human erythroid progenitor cells such as CFU-E also express both NMHC IIA and IIB. As shown in Figure 6A, PBMC that comprise monocytes and lymphocytes also exclusively express the IIA isoform. Platelets and granulocytes exclusively express the IIA isoform.18 Taken together, the presence of IIB isoform may be restricted to the erythroid lineage among hematopoietic cells, which may be an intriguing distribution of myosin II isoforms considering the characteristic feature of enucleation of erythroblasts.
We demonstrated that the proliferation of CFU-E was inhibited by the exogenous expression of the rod fragments for NMHC IIA or IIB, which suggests that the expressions of the rod fragments of NMHC IIA and IIB are functional in the inhibition of cell division of CFU-E. We further showed that the enucleation of mature erythroblasts was inhibited by the transfection of the rod fragment for myosin IIB. Because filament formation is necessary for myosin II to function, exogenous expression of rod fragments containing the critical regions for assembly could exhibit a dominant negative effect, preventing the normal assembly of endogenous myosin II. In fact, it was shown that a 72-kDa rod fragment of NMHC IIB acts as a dominant-negative form and induced aberrant cell shape.40 We also demonstrated that the myosin IIB rod fragment, BRF305 (Phe 1672–Glu 1976), can inhibit the function of endogenous myosin IIB by inhibiting normal filament assembly of MRC-5 SV1 TG1 cells, the SV40-transformant of human embryonic lung fibroblast MRC-5.32 Thus, our findings indicate that enucleation of human erythroblasts involves non-muscle myosin IIB. Information concerning the specific functions of myosin IIB has been increased by the studies of cells isolated from NMHC IIB knockout mice. Ablation of NMHC IIB resulted in survival to E14.5, but with marked abnormalities in the heart including a ventricular septal defect, abnormal positioning of the aorta, and abnormalities including hydrocephalus and the abnormal migration of certain groups of neurons.41-43 A defect in cytokinesis was observed in NMHC IIB−/− cardiac myocytes,42 possibly because myosin IIA is absent in these cells.
There is a possibility that the relative expression level of the rod fragment, ARF296, against endogenous myosin IIA is not sufficient to show an inhibitory effect32 in the enucleation of mature erythroblasts. We thus do not exclude the possibility that myosin IIA is also involved in enucleation of human erythroblasts. Interestingly, the clinical manifestations12 of MYH9-related diseases involve the lens, causing cataracts.18 The specific role of myosin IIA in maintaining transparency of the lens is unknown; however, Maddala et al44 showed that inhibition of myosin light chain kinase resulted in the development of nuclear lens opacity and abnormal fiber cell organization, suggesting an association between myosin IIA and lens cell enucleation.
Keerthivasan et al previously reported that blebbistatin failed to block enucleation, and suggested that contraction of the actomyosin ring is not essential for the nuclear expulsion of murine erythroblasts.10 However, we and others have demonstrated the presence of actomyosin contractile rings in the erythoblasts with incipient enucleation,5,6 which strongly suggests that the actomyosin ring is functional in enucleation. The reason for this discrepancy in the effects of blebbistatin between mouse and human erythroblasts remains unclear. The efficacy of inhibitors for cell division often varies depending on the species of the cell observed.19 Moreover, there are phenotypic differences in non-muscle myosin IIA deficiency between humans and mice. In humans, various mutations in the Myh9 gene that encodes the NMHC IIA cause autosomal dominant disease, whereas in mice the complete deficiency is embryonic lethal but heterozygous mice are nearly normal.18,45 One hypothesis might be that the degree of inhibition of non-muscle myosin II is more strictly required in mice to observe any effect of this molecule on the enucleation of erythroblasts.
Using human erythroblasts, we confirmed that the actin polymerization inhibitor cytochalasin D5 and the Rac-specific inhibitor NSC237666 completely blocked enucleation. Tubulin/kinesin inhibitors blocked cell division of CFU-E but did not inhibit the enucleation, which suggests that the process of erythroblast enucleation is not entirely the same as that of cytokinesis. In addition, this study contributes the novel finding that the Eg5 inhibitor monastrol also does not inhibit the final step of enucleation in human erythroblasts. Monastrol is a reversible, cell-permeable, non-tubulin-interacting inhibitor of the mitotic kinesin Eg5 motor protein.46,47 Eg5 is a member of the Kinesin-5 (BimC) subclass of kinesins and influences microtubules that form and organize the mitotic spindle in dividing cells.46,47 Mammalian cells exposed to monastrol in vitro progress normally through the S and G2 phases of the cell cycle, but are temporarily delayed during mitosis. This delay is accompanied by faulty chromosome separation and the presence of a monoastral spindle composed of a radial array of microtubules surrounded by a ring of chromosomes.48 Eg5 independent enucleation of human erythroblasts suggests that this is a process independent of the centrosome.
In conclusion, this study shows that the inhibition of ROCK and non-muscle myosin II ATPase completely blocked enucleation of human erythroblasts, demonstrating for the first time that non-muscle myosin II is required for human erythroblast enucleation. Further, we demonstrated that myosin IIB is involved in human erythroblast enucleation. An increased body of mechanistic knowledge about non-muscle myosin II isoforms in enucleation may help us to explore the unknown NMHC abnormalities in hematologic disorders.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Prof Mohandas Narla for helpful discussions and comments and to Keiko Iwamoto, Hiromi Kataho and Etsuko Kobayashi from the Department of Hematology, Nephrology, and Rheumatology, Akita University Graduate School of Medicine for their valuable technical assistance.
This study was supported in part by Grants-in-Aid (23591412), funds from the “Global Center of Excellence Program (COE)” of the Ministry of Education, Science, Technology, Sports, and Culture of Japan, and a research grant from the Idiopathic Disorders of Hematopoietic Organs Research Committee of the Ministry of Health, Labor and Welfare of Japan.
Authorship
Contribution: K.U. designed and performed experiments, analyzed data, and wrote the manuscript; Y.-M.G., M.T., M.H., Y.M., M.N., H.T., N.T. and A.K. performed experiments and helped to write the manuscript; W.N. and Y.T. analyzed and interpreted the data and helped to write the manuscript; and K.S. designed the study, interpreted data and helped to write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenichi Sawada, Department of Hematology, Nephrology, and Rheumatology, Akita University Graduate School of Medicine, Hondo 1-1-1, Akita 010-8543, Japan; e-mail: ksawada@doc.med.akita-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal