To the editor:
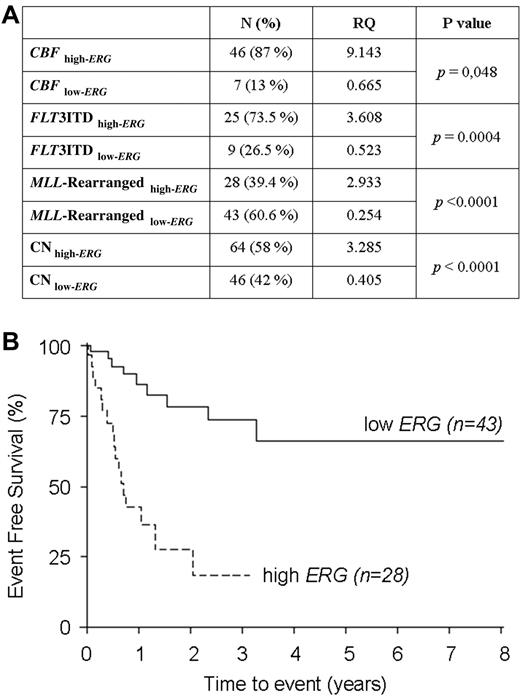
Childhood acute myeloid leukemia (AML) is a heterogeneous disease, in terms of genetic/molecular abnormalities resulting into marked differences in outcome.1 A myriad of proteins have been suggested aberrantly regulated in AML, and Ets-related gene (ERG, 21q22) expression in normal and aberrant hematopoiesis is currently under evaluation.2 High ERG expression has been associated with poor prognosis in cytogenetically normal adult AMLs.3,4 Only recently, Staffas et al reported that ERG expression was associated with an inferior probability of event-free survival (EFS) in northern European children with AML, although not as independent prognostic factor.5 We measured ERG expression in a cohort of 268 Italian pediatric patients (141 males and 127 females, median age at diagnosis 6 years, range 7 days-22 years) with newly diagnosed de novo AML enrolled in AIEOP LAM-2002 protocol.6 ERG expression, relative to ABL, was measured by real-time quantitative–PCR and calculated the relative quantity (RQ) by the comparative ΔΔCt method. ERG expression turned out to be higher (high-ERG, RQ > 1) or lower (low-ERG, RQ < 1) in AML patients, dividing them into 2 groups significantly different (N high-ERG = 162, RQ = 4927, N low-ERG = 106, RQ = 0371, P = .007). Patients were then divided into cytogenetic groups. Of these, 157 carried well-known molecular markers, such as Core Binding Factor (CBF) anomalies (n = 52), FLT3-ITD (n = 34) and the mixed-lineage leukemia gene (MLL) rearrangements (n = 71), whereas the remaining 111 patients were cytogenetically normal (CN). ERG was found to be different expressed between each AML subgroups, with statistical significance for all the subgroups analyzed (Figure 1A, Student t test, statistical significance P < .05). Overall Survival (OS), defined as the time from diagnosis to last follow-up or death, was measured. Results showed no statistical difference in OS for CN (Kaplan-Meier log-rank test, P = .58), CBF (P = .82), nor FLT3ITD (P = .54) patients according to ERG expression. By contrast, OS and EFS for patients with MLL-rearrangements and high-ERG expression were found to be significantly worse than those of patients with low-ERG (P < .001, Figure 1B) expression. Multivariate analysis by Cox regression model confirmed that high-ERG expression is an independent prognostic factor for EFS in MLL-rearranged patients (hazard risk = 4.22, 95% CI = 1.10-16.18, P = .036). In multivariate analysis complex karyotype remained the only other independent adverse factor in this group (P = .028, HR = 3.51, 95%CI = 1.15-10.74). These data indicate that ERG expression strongly influences the probability of OS and EFS of MLL-rearranged patients. Since childhood MLL-rearranged AML includes patients with marked differences in biology and outcome,7,8 we suggest the use of ERG expression to stratify patient's prognostic risk and to tailor therapeutic approaches.
ERG expression in AML influences EFS of MLL-rearranged patients. (A) ERG expression in AML subgroups. ERG expression relative to ABL housekeeping gene was measured by real-quantitative PCR. RQ is the relative quantity of ERG expression with respect to the average of its expression in healthy bone marrows (N = 17) calculated by comparative ΔΔCt method. CBF indicates core binding factor rearrangements; and CN, cytogenetically normal. (B) Probability of event-free survival in children with MLL-rearranged AML according to ERG expression. Event-free survival (EFS) for patients MLL-rearranged with high (73.3%) vs low (18.3%) ERG expression.
ERG expression in AML influences EFS of MLL-rearranged patients. (A) ERG expression in AML subgroups. ERG expression relative to ABL housekeeping gene was measured by real-quantitative PCR. RQ is the relative quantity of ERG expression with respect to the average of its expression in healthy bone marrows (N = 17) calculated by comparative ΔΔCt method. CBF indicates core binding factor rearrangements; and CN, cytogenetically normal. (B) Probability of event-free survival in children with MLL-rearranged AML according to ERG expression. Event-free survival (EFS) for patients MLL-rearranged with high (73.3%) vs low (18.3%) ERG expression.
Authorship
Acknowledgments: The authors thank all Italian AIEOP centers. They also thank Sabrina Gelain, Claudia Tregnago, Samuela Francescato, Maria Grazia Giacometti, Silvia Disarò and Katia Polato for their collaboration.
This study was supported by grants from Fondazione Città della Speranza-Padova, University of Padova, Istituto Superiore di Sanitá, Fondazione Veneto Banca and AIL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martina Pigazzi, Pediatrics Department, University of Padova-Città della, Speranza, Hematology-Oncology Laboratory via Giustiniani 3, 35 128 Padova-Italy; e-mail: martina.pigazzi@unipd.it.