Abstract
An important mediator of cytokine signaling implicated in regulation of hematopoiesis is the PI3K/protein kinase B (PKB/c-Akt) signaling module. Constitutive activation of this signaling module has been observed in a large group of leukemias. Because activation of this signaling pathway has been demonstrated to be sufficient to induce hematologic malignancies and is thought to correlate with poor prognosis and enhanced drug resistance, it is considered to be a promising target for therapy. A high number of pharmacologic inhibitors directed against either individual or multiple components of this pathway have already been developed to improve therapy. In this review, the safety and efficacy of both single and dual-specificity inhibitors will be discussed as well as the potential of combination therapy with either inhibitors directed against other signal transduction molecules or classic chemotherapy.
PI3K plays a critical role in both HSC maintenance and lineage development
The PI3K family consists of 3 distinct subclasses of which, to date, only the class I isoforms have been implicated in regulation of hematopoiesis. Three distinct catalytic class IA isoforms have been identified: p110α, p110β, and p110δ.1 These isoforms are predominantly activated by protein tyrosine kinases and form heterodimers with a group a regulatory adapter molecules, including p85α, p85β, p50α, p55α, and p55γ.1 In addition, a single-class 1B isoform termed p110γ has been described, which can be specifically activated by G-protein coupled receptors and associates with a p101 regulatory molecule.1 The most important substrate for these class I PI3Ks is phosphatidylinositol 4,5 bisphosphate [PI(4,5)P2] which can be phosphorylated at the D3 position of the inositol ring on extracellular stimulation, resulting in the formation of phosphatidylinositol 3,4,5 trisphosphate [PI(3,4,5)P3]1 (Figure 1). The activity of PI3K can be inhibited by phosphate and tensin homologue (PTEN), a ubiquitously expressed tumor suppressor protein that can dephosphorylate PIP3, resulting in the formation of PI(4,5)P2.1,2 Similarly, SH2-containing inositol-5′-phosphatase 1 (SHIP1), a protein predominantly expressed in hematopoietic cells, can hydrolyze PIP3 to generate PI(3,4)P2.1,2 Although both PTEN and SHIP1 act on the main product of PI3K, PI(3,4,5)P3, the generated products PI(3,4)P2 and PI(4,5)P2 both act as discrete second messengers activating distinct downstream events.2 Recently, pleckstrin homology (PH) domain leucine-rich repeat protein phosphatase (PHLPP) was found to terminate PKB signaling by directly dephosphorylating and inactivating PKB.3 However, a role for PHLPP in regulation of hematopoiesis remains to be determined. Inositol 1,3,4,5-tetrakiphosphate [Ins(1,3,4,5)P4], which is generated from inositol 1,4,5-triphosphate [Ins(1,4,5)P3] by inositol triphosphate 3-kinase B (InsP3KB), is considered to be a fourth negative regulator of the PI3K/PKB signaling module. The activity of downstream effectors of PI3K, including PKB, can be abrogated because of binding of PI(3,4,5)P3-specific pleckstrin homology (PH) domains to Ins(1,3,4,5)P4.4 Since the identification of PI3K, it has become evident that it plays an essential role in proliferation, survival, and differentiation of many different cell types. Correct regulation of the activity of PI3K has been demonstrated to be essential for maintenance of hematopoietic stem cells using PTEN- and SHIP1-deficient mice that displayed constitutive activation of PI3K activity. In those mice, an initial expansion of HSCs could be observed that was followed by a depletion of long-term repopulating HSCs.5 The role of class I PI3K isoforms in lineage development has been investigated in more detail. Combined deletion of p85α, p55α, and p50α has, for example, resulted in a complete block in B lymphocyte development.6 Similarly, introduction of a mutated, catalytically inactive p110δ (p110δD910A) in the normal p110δ locus also resulted in a block in early B lymphocyte development, whereas T-cell development was unaffected.7 These results indicate that PI3K activity is essential for normal B lymphocyte development. In addition, conditional deletion of either PTEN or SHIP1 in adult HSCs, resulting in activation of the PI3K pathway, not only reduced the level of B-lymphocytes but also enhanced the level of myeloid cells.8,9 Furthermore, enhanced levels of megakaryocyte progenitors have been observed in SHIP1-deficient mice.10 In time, both PTEN- and SHIP1-deficient mice developed a myeloproliferative disorder that progressed to leukemia.8,9 In PTEN heterozygote (+/−) SHIP null (−/−) mice, a more severe myeloproliferative phenotype, displayed by reduced erythrocyte and platelet numbers and enhanced white blood cell counts including elevated levels of neutrophils and monocytes in the peripheral blood, could be observed.11 Recently, a shorter SHIP1 isoform (s-SHIP1), which is transcribed from an internal promoter in the SHIP1 gene, has also been implicated in positive regulation of lymphocyte development during hematopoiesis.12 Pharmacologic inhibition of PI3K activity in human umbilical cord blood-derived CD34+ HSCs and progenitor cells revealed that inhibition of the activity of PI3K is sufficient to completely abrogate both proliferation and differentiation of eosinophil and neutrophil progenitors eventually leading to cell death.13 Finally, mice deficient for InsP3KB, resulting in an induction of PKB activity, display higher levels of granulocyte-macrophage progenitors and mature neutrophils in the bone marrow14 and dramatically reduced levels of mature CD4+ and CD8+ T lymphocytes.15 Together, these studies suggest that correct temporal regulation of PI3K activity is critical for both HSC maintenance and regulation of lineage development.
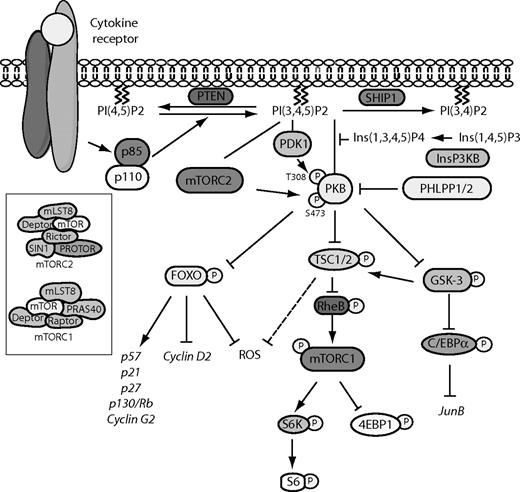
Schematic representation of the PI3K/PKB signaling module. Activation of PI3K by receptor stimulation results in the production of PtdIns(3,4,5)P3 at the plasma membrane. PKB subsequently translocates to the plasma membrane where it is phosphorylated by PDK1 and the mTORC2 complex. On phosphorylation, PKB is released into the cytoplasm where it can both inhibitory phosphorylate multiple substrates, including FoxO transcription factors and GSK-3, and induce the activity of other substrates, such as mTOR as part of the mTORC1 complex. Negative regulators of the PI3K/PKB signaling module include PTEN, SHIP1, Ins(1,3,4,5)P4, and PHLPP1/2.
Schematic representation of the PI3K/PKB signaling module. Activation of PI3K by receptor stimulation results in the production of PtdIns(3,4,5)P3 at the plasma membrane. PKB subsequently translocates to the plasma membrane where it is phosphorylated by PDK1 and the mTORC2 complex. On phosphorylation, PKB is released into the cytoplasm where it can both inhibitory phosphorylate multiple substrates, including FoxO transcription factors and GSK-3, and induce the activity of other substrates, such as mTOR as part of the mTORC1 complex. Negative regulators of the PI3K/PKB signaling module include PTEN, SHIP1, Ins(1,3,4,5)P4, and PHLPP1/2.
PKB is an important mediator of PI3K in regulation of hematopoiesis
An important mediator of PI3K signaling is protein kinase B (PKB/c-akt). Whereas PKB is cytosolic in unstimulated cells, PI3K-mediated activation of PKB requires translocation of PKB to the membrane. PI(3,4,5)P3, which is generated by PI3K, serves as an anchor for PH domain–containing proteins in the membrane, including PKB.16 Activation of PKB requires phosphorylation on both Thr308 in the activation loop, by phosphoinositide-dependent kinase 1 (PDK1) and Ser473, within the carboxyl-terminal hydrophobic motif, by the MTORC2 complex that consists of multiple proteins, including mTOR, Rictor, mLST8, DEPTOR, Sin1, and PROTOR17 (Figure 1).
Three highly homologous PKB isoforms have been described to be expressed in mammalian cells: PKBα, PKBβ, and PKBγ. PKB has been demonstrated to play an important role in regulation of cell survival and proliferation in a large variety of cell types.18 Analysis of HSCs derived from PKBα/PKBβ double-knockout mice revealed that PKB plays an important role in maintenance of long-term repopulating HSCs. These PKBα/PKBβ double-deficient HSCs were found to persist in the G0 phase of the cell cycle, suggesting that the functional defects observed in these mice with regard to long-term hematopoiesis were caused by enhanced quiescence.19 In contrast, loss of only one of the isoforms only minimally affected HSCs.19 In addition, ectopic expression of constitutively active PKB in mouse HSCs conversely resulted in transient expansion and increased cycling of HSCs, followed by apoptosis and expansion of immature progenitors in BM and spleen, which was also associated with impaired engraftment.20 In addition to HSC maintenance, PKB also plays a critical role in the regulation of cell fate decisions during hematopoietic lineage development. High PKB activity in human umbilical cord blood-derived hematopoietic progenitors was, for example, found to promote neutrophil and monocyte differentiation and to inhibit B lymphocyte development, whereas reduction of PKB activity has been demonstrated to be required for optimal eosinophil maturation.13 In addition, PKB plays an important role in regulation of proliferation and survival, but not maturation, of human dendritic cell (DC) progenitors.21 The development of a myeloproliferative disease, characterized by extramedullary hematopoiesis in liver and spleen, and lymphoblastic thymic T-cell lymphoma in the majority of mice transplanted with mouse bone marrow cells ectopically expressing constitutively active PKB further demonstrates the importance for correct regulation of PKB activity in HSCs and progenitor cells.20 In addition, recent findings suggest that constitutive activation of PKBβ is sufficient to accelerate MYC-induced T-cell acute lymphoblastic leukemia (T-ALL) in zebrafish.22 Furthermore, analysis of mice deficient for both PKBα and PKBβ, but not single knockout mice, revealed that the generation of marginal zone and B1 B cells and the survival of mature follicular B cells also depend on correct regulation of PKB activity.23
Downstream effectors of PKB differentially regulate hematopoiesis
Multiple PKB substrates have been identified, including members of the FoxO subfamily of forkhead transcription factors FoxO1, FoxO3, and FoxO4, the serine/threonine kinase glycogen synthase kinase-3 (GSK-3), and the serine/threonine kinase mammalian target of rapamycin (mTOR) as part of the MTORC1 complex, which also includes the regulatory associated protein of mTOR (Raptor; Figure 1).18 FoxO transcription factors, which are inhibitory phosphorylated by PKB,18 are known to play an important role in regulation of proliferation and survival of various cell types. In addition, these transcription factors play an important role in HSC maintenance, which has been demonstrated by conditional deletion of FoxO1, FoxO3, and FoxO4 in the adult hematopoietic system.24 An initial expansion of HSCs has been observed in those mice, which correlated with an HSC-specific up-regulation of cyclin D2 and down-regulation of cyclin G2, p130/Rb, p27, and p21.24 After this initial expansion, a reduction in HSC numbers was observed, resulting in a defective long-term repopulating capacity.24 Similarly, competitive repopulation experiments revealed that deletion of FoxO3 alone is also sufficient to impair long-term reconstitution.25 In addition, in aging mice deficient for FoxO3, the frequency of HSCs was increased compared with wild-type littermate controls.25 Both mouse models have also been used to examine the role of FoxO transcription factors in lineage development. Conditional deletion of FoxO1, FoxO3, and FoxO4 resulted in enhanced levels of myeloid cells and decreased numbers of peripheral blood lymphocytes under normal conditions. In time, these mice developed leukocytosis characterized by a relative neutrophilia and lymphopenia.24 In contrast, in FoxO3-deficient mice, neutrophilia only developed during aging and under myelosuppressive stress conditions.26 Although deletion of FoxO3 alone was not sufficient to induce neutrophilia, ectopic expression of a constitutively active, nonphosphorylatable, FoxO3 mutant in mouse hematopoietic progenitors did result in a decrease in the formation of both myeloid and erythroid colonies,27 suggesting that FoxO3 does play an important role in lineage development. Modulation of the activity of either PKB or FoxO transcription factors has been observed to alter the level of reactive oxygen species (ROS). Although ROS levels are reduced in PKBα/β-deficient mice,19 increased levels have been observed in mice deficient for FoxO.25 Increasing the ROS levels in PKBα/β-deficient mice was sufficient to rescue differentiation defects, but not impair long-term hematopoiesis.19 Reducing the ROS levels in FoxO-deficient mice with N-acetyl-L-cysteine, an antioxidative agent, was sufficient to abrogate the enhanced levels of proliferation and apoptosis in FoxO-deficient HSCs and to restore the reduced colony-forming ability of these cells.24 These studies demonstrate that correct regulation of ROS by FoxO transcription factors is essential for normal hematopoiesis.
Recent findings have demonstrated that correct regulation of the activity of GSK-3, which is inhibitory phosphorylated by PKB,18 is also essential for HSC maintenance. A reduction in long-term, but not short-term, repopulating HSCs has, for example, been observed in GSK3-deficient mice.28 In addition, disruption of GSK-3 activity in mice with a pharmacologic inhibitor or shRNAs has been shown to transiently induce expansion of both HSCs and progenitor cells followed by exhaustion of long-term repopulation HSCs.28,29 An acceleration of neutrophil and megakaryocyte recovery could be observed after transplantation of mice treated with a GSK-3 inhibitor, suggesting a role for GSK-3 in lineage development.29 Indeed, ex vivo experiments with human hematopoietic progenitors revealed that GSK-3 can enhance eosinophil differentiation and inhibit neutrophil development at least in part via regulation of C/EBPα, a key regulator of hematopoiesis.13
A third, important mediator of PI3K/PKB signaling is mTOR.18 In contrast to GSK-3 and FoxO transcription factors, which are inhibitory phosphorylated by PKB, the activity of mTOR is positively regulated by PKB. Inhibition of the GTPase activating protein Tuberous sclerosis protein 2 (TSC2)/TSC1 complex by PKB results in accumulation of GTP-bound Rheb and subsequent activation of mTOR.30 In addition, because rapamycin was sufficient to revert the HSC phenotype of mice in which GSK-3 was depleted, it is probable that mTOR is an important effector of GSK-3.28 Conditional deletion of TSC1 in mice, resulting in activation of mTOR, has been demonstrated to enhance the percentage of cycling HSCs and to reduce the self-renewal capacity of HSCs in serial transplantation assays.31 In addition, treatment of PTEN-deficient mice with rapamycin appears to be sufficient to revert the HSC phenotype in those mice, indicating that mTORC1 is an important mediator of PI3K in regulation of HSC proliferation.32 In addition, deletion of TSC1 has been demonstrated to induce ROS levels in HSCs. In vivo treatment of TSC1-deficient mice with an ROS antagonist restored HSC numbers and function,31 suggesting that TSC1, similar to FoxO transcription factors, regulates HSC numbers at least in part via ROS. Although mice deficient of TSC1 also display reduced granulocyte and lymphocyte numbers, this is probably because of reduced progenitor proliferation. In contrast to PKB, which regulates both proliferation and differentiation of myeloid progenitors,13 mTOR primarily regulates expansion of hematopoietic progenitors, as was demonstrated for, for example, granulocyte progenitors33 and progenitors for interstitial DCs and Langerhans cells.21 Although the molecular mechanisms underlying mTOR-mediated regulation of lineage development are largely unknown, it has recently been demonstrated that mTOR decreases the ratio of wild-type C/EBPα (C/EBPαp42) and truncated C/EBPαp30, which is generated by alternative translation initiation, resulting in high levels of the smaller p30 C/EBPα isoform,34 which inhibits trans-activation of C/EBPα target genes in a dominant-negative manner and binds to the promoters of a unique set of target genes to suppress their transcription.
Together, these studies show that FoxO transcription factors GSK-3 and mTOR are all important mediators of PI3K in terms of HSC maintenance and lineage development.
Deregulated PI3K/PKB signaling in malignant hematopoiesis
The studies described in the first three sections of this review clearly demonstrate that deregulation of the PI3K/PKB signaling module dramatically affects hematopoiesis resulting in the development of hematologic malignancies. To investigate whether, in patients, the development of leukemia can be caused by aberrant regulation of this signaling module, research has focused on identifying mutations in PI3K and upstream and downstream regulators of this molecule (Figure 2). Constitutive activation of class I PI3K isoforms has been observed in a high percentage of patients with acute35,36 and chronic leukemia.37 In contrast to the expression of p110α, β, and γ, which is only up-regulated in leukemic blasts of some patients, p110δ expression appears to be consistently up-regulated in cells from patients with either acute myeloid leukemia (AML) or acute promyelocytic leukemia.36,38 Activating mutations in the kinase domain (H1047R) and helical domain (E545A) of p110α have been detected in a wide variety of human solid tumors.39 The latter has also been detected in acute leukemia, albeit in a very low percentage (1 of 88).39 In addition, another activating helical domain mutation (E545G) has recently been detected in a chronic myeloid leukemia (CML) cell line.40 Furthermore, both activating mutations in p110α (PIK3CA) and in-frame insertions/deletions in the PI3K regulatory subunit p85α (PIK3R1) have been observed in a low number of pediatric T-ALL patients (both 2 of 44).41 In a small group of myelodysplastic syndrome (MDS) patients, however, the above described mutations could not be detected.42
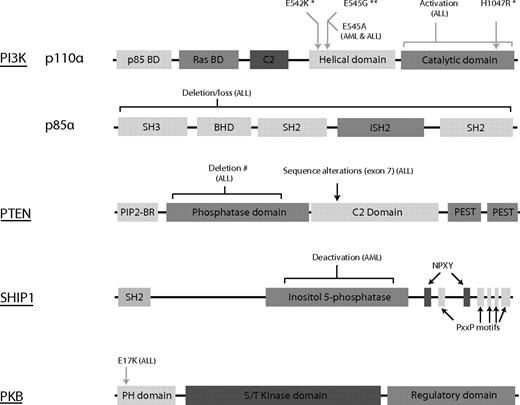
Schematic representation of the known mutations in the PI3K/PKB signaling module in leukemia. Aberrant regulation of the PI3K/PKB signaling module has been found in a large group of patients with leukemia. However, only few mutations are known to directly affect hematopoiesis. Mutations indentified in leukemic cells are indicated above the linear (unscaled) representations of PI3K, SHIP, PTEN, and PKB. Grey arrows/lines indicate activating mutations; black arrows, inactivating mutations/deletions. *Mutations found in AML and ALL cell lines. **Mutation found in CML cell line. #Mutations inducing leukemia in mouse model. BD indicates binding domain; C2, C2 domain (putative membrane binding domain); SH2, Src homology 2 region (tyrosine-phosphorylated residue binding region); BHD, Bcr homology domain; SH3, Src homology 3 region (proline-rich protein-protein interaction region); and BR, binding region.
Schematic representation of the known mutations in the PI3K/PKB signaling module in leukemia. Aberrant regulation of the PI3K/PKB signaling module has been found in a large group of patients with leukemia. However, only few mutations are known to directly affect hematopoiesis. Mutations indentified in leukemic cells are indicated above the linear (unscaled) representations of PI3K, SHIP, PTEN, and PKB. Grey arrows/lines indicate activating mutations; black arrows, inactivating mutations/deletions. *Mutations found in AML and ALL cell lines. **Mutation found in CML cell line. #Mutations inducing leukemia in mouse model. BD indicates binding domain; C2, C2 domain (putative membrane binding domain); SH2, Src homology 2 region (tyrosine-phosphorylated residue binding region); BHD, Bcr homology domain; SH3, Src homology 3 region (proline-rich protein-protein interaction region); and BR, binding region.
Importantly, ectopic expression of mutated p110α has been demonstrated to be sufficient to induce leukemia in a mouse transplantation model.43 Alternatively, constitutive activation of PI3K may also be caused by either aberrant expression or activation of PTEN. Reduced expression of PTEN has, for example, been observed in different types of leukemia. Although analysis of both myeloid leukemic cell lines and primary AML blasts indicates that PTEN mutations are rare in AML,44,45 both homozygous and heterozygous deletion of PTEN as well as nonsynonymous sequence alterations in exon 7 have been detected in approximately 15% and 25% of T-ALL patients, respectively.41 In addition, analysis of primary T-ALL cells revealed that, as a result of mutation-induced alternative splicing, full-length SHIP1 expression is often low or undetectable.46 In addition, an inactivating mutation in the phosphatase domain of SHIP1 has also been detected in primary AML blasts.47
Although constitutive activation of PKB, resulting in enhanced survival signals,48-50 has been demonstrated in a significant fraction of AML51,52 and chronic lymphoid leukemia (CLL) patients,49,53,54 until recently, no PKB mutations were found in patients with leukemia. However, an activating mutation in the pleckstrin homology domain of PKB (E17K) has recently been detected in various solid tumors55 and one pediatric T-ALL patient.41 In contrast, this mutation could not be detected in a several cohorts of CLL,56,57 CML,58 and AML patients,57,59 suggesting that this particular mutation is very rare. However, transplantation of mice with bone marrow cells ectopically expressing this E17K mutation was sufficient to induce leukemia.55 Although mutations in mTOR have thus far not been described in hematologic malignancies, a recent study has identified several mTOR mutations in solid tumors.60 Interestingly, 2 single amino acid mutations, S2215Y and R2505P, identified in large intestine adenocarcinoma and renal cell carcinoma, respectively, appear to induce constitutive activation of mTOR, even under nutrient starvation conditions.60
In addition to mutations in the PI3K signaling module itself, mutations in cytokine receptors, including constitutive activation of FMS-like tyrosine kinase 3 (FLT3) by internal tandem duplication (Flt3-ITD)61 and mutation in c-Kit43,62 have been described to affect PI3K signaling in hematologic malignancies. In addition to these tyrosine kinase receptors, the activity of the PI3K/PKB pathway can also be enhanced by several fusion proteins, including Bcr-Abl,63 which can be detected in virtually all patients with CML and in a subset of patients with ALL.64 The mechanisms underlying Bcr-Abl–mediated activation of the PI3K/PKB signaling pathway have been investigated extensively. Coimmunoprecipitation experiments revealed that Bcr-Abl associates with Shc,65 which subsequently binds to the p85α subunit of PI3K66 resulting in activation. In addition, expression of Bcr-Abl has been shown to reduce the levels of PHLPP1 and PHLPP2, which are negative regulators of PKB phosphorylation,67 and to up-regulate the level of p110γ.68 Bcr-Abl has also been shown to indirectly induce the activity of the PI3K/PKB signaling module by, for example, increasing the level of Nox-4–generated ROS resulting in inhibition of PP1a, a negative regulator of PI3K/PKB.69
Other potential regulators of PI3K often mutated or aberrantly expressed in leukemia include Ras,1 Evi1,70 PP2A,52 and casein kinase 2 (CK2).35 Inhibition of CK2 has been shown to reduce the activity of the PI3K/PKB signaling module by dephosphorylation and activation of PTEN, resulting in induction of apoptosis in primary CLL cells in vitro.71,72 Interestingly, in a subset of MDS patients, it was demonstrated that deletion of glutathione S-transferase θ can result in the generation of a DNA sequence homologous to mTOR, thereby inducing the activity of downstream effectors of mTOR in a PI3K/PKB-independent manner. Inhibition of mTOR in these cells was sufficient to induce apoptosis.73,74
It is evident that the activity of the PI3K/PKB signaling module is regulated by various extracellular factors, suggesting that deregulation of the production of those factors by components of the microenvironment could also result in aberrant hematopoiesis. Several studies have revealed that the microenvironment of CLL patients constitutively activates PI3K in leukemic cells, induces proliferation and survival of CLL cells, and contributes to the resistance of leukemic cells to cytotoxic agents.75-80 Inhibition of the PI3K/PKB signaling pathway was shown to be sufficient to induce apoptosis in CLL cells.75,76 In addition, the migratory capacity of primary CLL cells has been demonstrated to be enhanced by CX3CL1 in an autocrine manner.78 Constitutive release of soluble CX3CL1 by CLL cells results in a PKB-dependent up-regulation of CXCR4, a chemokine receptor that is important for CLL cell migration.78
Although the studies described in this section clearly demonstrate that constitutive activation of the PI3K/PKB signaling module can result in the development of leukemia, enhanced FoxO activity has also been observed in 40% of the AML patients.81 Similarly, enhanced FoxO activity could also be observed in MLL-AF9–induced AML in a mouse model.81 Activation of PKB or deletion of FoxO1/3/4 was sufficient to reduce leukemic cell growth, suggesting that inactivation of the PI3K/PKB signaling module contributes to the development of MLL-AF9–induced leukemia.81 Together, these studies demonstrate that, in addition to constitutive activation of the PI3K/PKB signaling pathway, inactivation of this module can also result in the development of leukemia.
Prognosis of leukemia with activated PI3K/PKB signaling
Because the PI3K/PKB signaling module is aberrantly regulated in many patients with leukemia, the question was raised whether the level of PI3K/PKB activation in leukemic blasts could be used as a prognostic marker. Mouse transplantation studies with blasts from pediatric de novo B-ALL patients revealed that a rapid induction of leukemia correlates with enhanced mTOR activity in the leukemic blasts.82 In addition, comparison of pediatric T-ALL patients revealed that the survival rate of patients positively correlates with the level of PTEN.83 Similar observations were made in a different cohort of pediatric T-ALL patients, in which PTEN deletions correlated with early treatment failure in T-ALL.41 Furthermore, constitutive activation of the PI3K pathway, as measured by enhanced FoxO3 expression84 or phosphorylation,85 enhanced levels of phosphorylated, and therefore inactive, PTEN86 or enhanced levels of phosphorylated PKB52,87 is also considered to be an independent adverse prognostic factor in AML patients. Similarly, in MDS, activation of the PI3K/PKB pathway has only been detected in high-risk MDS, but not in low-risk MDS patients,88,89 suggesting that high PKB activity may also be an adverse prognostic factor for MDS.
In contrast, Tamburini et al suggest that enhanced PKB phosphorylation positively correlates with the survival of AML patients.90 Although the short-term survival rate (within 12 months) appeared to be slightly lower in the group displaying high PKB phosphorylation compared with the group with low levels of phosphorylated PKB, both the long-term survival and relapse-free survival were significantly enhanced.90 Except for this last study, all other studies suggest that enhanced PI3K/PKB activity correlates with reduced survival rate in both ALL and AML patients.
The molecular mechanisms underlying this reduced prognosis are, thus far, incompletely understood. However, a reduced apoptotic response has been observed in AML blasts displaying enhanced PI3K/PKB activation.91 In addition, PI3K has been demonstrated to induce expression of the multidrug resistance-associated protein 1, a member of the ATP-binding cassette membrane transporters that functions as a drug efflux pump,92 suggesting that high PI3K activity induces drug resistance. The observation that high levels of multidrug resistance-associated protein 1 correlate with enhanced drug resistance in AML cells and poor prognosis supports that hypothesis.93
PI3K/PKB signaling as therapeutic target in leukemia
Because aberrant regulation of the PI3K signaling module has frequently been observed in leukemic cells, PI3K and its downstream effectors are considered to be promising targets for therapy (Figure 3; Table 1). The functionality of LY294002 and wortmannin, 2 well-known PI3K inhibitors that prevent ATP to bind to and activate PI3K, has for example been investigated extensively. Preclinical experiments indicate that both LY294002 and wortmannin induce apoptosis in leukemic cells and rescue drug sensitivity. However, it has also been demonstrated that both inhibitors are detrimental for normal cells.13,94 In addition, both inhibitors exhibit little specificity within the PI3K family and inhibit the activity of other kinases, including CK2 and smMLCK, respectively.95 Recently, several novel PI3K inhibitors, including S14161,96 the p110α-selective inhibitor AS702630,97 the p110β-selective inhibitor TGX-115,36 and the p110δ inhibitors IC8711436,38 and CAL-10180,98-100 have been discovered that also appear to affect proliferation and survival of leukemic blasts. It has, for example, been demonstrated that IC87114 reduces proliferation and survival of both AML blasts38 and acute promyelocytic leukemia cells36 without affecting the proliferation of normal hematopoietic progenitors.38 For CAL-101, a dual mechanism of action has been revealed in patients with CLL. CAL-101 both decreases the survival of CLL cells directly and abrogates cellular interactions between CLL cells components of the tissue microenvironment.99,100 Phase 1 or 2 clinical trials (NCT00710528 and NCT01090414) have therefore been initiated to examine the efficacy of CAL-101 as a therapeutic agent in CLL.
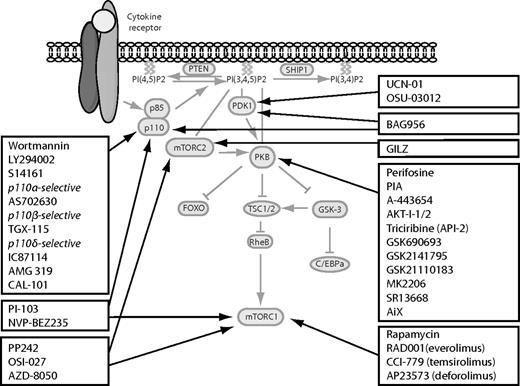
Schematic representation of the PI3K/PKB signaling module and the available inhibitors inhibiting this pathway. Several inhibitors have been developed to inhibit aberrant regulation of the PI3K/PKB signaling module in leukemia. These inhibitors target single or multiple proteins in the pathway. Black boxes represent groups of similar inhibitors; and black arrows, the specific target of the inhibitors.
Schematic representation of the PI3K/PKB signaling module and the available inhibitors inhibiting this pathway. Several inhibitors have been developed to inhibit aberrant regulation of the PI3K/PKB signaling module in leukemia. These inhibitors target single or multiple proteins in the pathway. Black boxes represent groups of similar inhibitors; and black arrows, the specific target of the inhibitors.
Inhibitors of PI3K/PKB signaling pathway
| Target . | Compound . | Effect . | Clinical trials (phase) . | Leukemia . | Reference(s) . | |
|---|---|---|---|---|---|---|
| In vitro . | In vivo . | |||||
| PI3K | Wortmannin | + | − | 147,148 | ||
| LY294002 | + | − | 37,148,–150 | |||
| AS702630 | + | − | 105 | |||
| S14161 | + | + | 96 | |||
| p110β | TGX-115 | + | − | 36,105 | ||
| p110δ | IC87114 | + | − | 36,38,105,145 | ||
| AMG 319 | − | − | NCT01300026 (1) | CLL and ALL | ||
| CAL-101 | + | + | NCT01090414 (1) | CLL and AML | 80,98,99 | |
| + | + | NCT00710528 (1) | CLL and AML | 80,98,99 | ||
| PDK1 | UCN-01 | + | − | 122 | ||
| PKB | Perifosine | + | − | NCT00391560 (2) | RRL | 101,–103 |
| − | − | NCT00873457 (2) | CLL | |||
| PIA | + | − | 104 | |||
| A-443654 | + | − | 53 | |||
| AiX | + | − | 107 | |||
| Triciribine (API-2) | + | − | NCT00363454 (1) | 106 | ||
| AKT-I-1/2 | + | − | 52,53 | |||
| GSK690693 | + | − | NCT00493818 (1) | 151 | ||
| MK2206 | − | − | NCT01231919 (1) | RRL | ||
| − | − | NCT01253447 (2) | AML | |||
| SR13668 | − | − | NCT00896207 (1) | |||
| GSK2141795 | − | − | NCT00920257 (1) | |||
| GSK21110183 | − | − | NCT00881946 (1/2) | RRL | ||
| mTOR | Rapamycin | + | + | NCT00795886 (2) | ALL | 112,152,153 |
| + | + | NCT00068302 (1) | ALL and AML | 112,152,153 | ||
| + | + | (2) | CML | 154,155 | ||
| RAD001 | + | + | NCT00636922 (1) (1/2) | AML AML and MDS | 138 | |
| + | + | (2) | B-CLL | 109 | ||
| + | + | (2) | CLL | 116 | ||
| CCI-779 | + | + | NCT00290472 (2) | CLL | ||
| + | + | NCT00084916 (2) | RRL | |||
| + | + | NCT00084474 (2) | CLL | |||
| + | + | (2) | ALL | 156 | ||
| AP23573 | − | − | (2) | AML | 113 | |
| NCT00086125 (2) | RRL | |||||
| PP242 | + | + | 117,118,157 | |||
| OSI-027 | + | − | 118,–120 | |||
| AZD-8050 | + | − | 118 | |||
| PI3K/mTOR | PI-103 | + | + | 76,126,127,158 | ||
| NVP-BEZ235 | + | + | 123,–125 | |||
| PI3K/PDK1 | BAG956 | + | + | 121 | ||
| PKB/PDK1/Flt3 | KP372-1 | + | − | 128 | ||
| Target . | Compound . | Effect . | Clinical trials (phase) . | Leukemia . | Reference(s) . | |
|---|---|---|---|---|---|---|
| In vitro . | In vivo . | |||||
| PI3K | Wortmannin | + | − | 147,148 | ||
| LY294002 | + | − | 37,148,–150 | |||
| AS702630 | + | − | 105 | |||
| S14161 | + | + | 96 | |||
| p110β | TGX-115 | + | − | 36,105 | ||
| p110δ | IC87114 | + | − | 36,38,105,145 | ||
| AMG 319 | − | − | NCT01300026 (1) | CLL and ALL | ||
| CAL-101 | + | + | NCT01090414 (1) | CLL and AML | 80,98,99 | |
| + | + | NCT00710528 (1) | CLL and AML | 80,98,99 | ||
| PDK1 | UCN-01 | + | − | 122 | ||
| PKB | Perifosine | + | − | NCT00391560 (2) | RRL | 101,–103 |
| − | − | NCT00873457 (2) | CLL | |||
| PIA | + | − | 104 | |||
| A-443654 | + | − | 53 | |||
| AiX | + | − | 107 | |||
| Triciribine (API-2) | + | − | NCT00363454 (1) | 106 | ||
| AKT-I-1/2 | + | − | 52,53 | |||
| GSK690693 | + | − | NCT00493818 (1) | 151 | ||
| MK2206 | − | − | NCT01231919 (1) | RRL | ||
| − | − | NCT01253447 (2) | AML | |||
| SR13668 | − | − | NCT00896207 (1) | |||
| GSK2141795 | − | − | NCT00920257 (1) | |||
| GSK21110183 | − | − | NCT00881946 (1/2) | RRL | ||
| mTOR | Rapamycin | + | + | NCT00795886 (2) | ALL | 112,152,153 |
| + | + | NCT00068302 (1) | ALL and AML | 112,152,153 | ||
| + | + | (2) | CML | 154,155 | ||
| RAD001 | + | + | NCT00636922 (1) (1/2) | AML AML and MDS | 138 | |
| + | + | (2) | B-CLL | 109 | ||
| + | + | (2) | CLL | 116 | ||
| CCI-779 | + | + | NCT00290472 (2) | CLL | ||
| + | + | NCT00084916 (2) | RRL | |||
| + | + | NCT00084474 (2) | CLL | |||
| + | + | (2) | ALL | 156 | ||
| AP23573 | − | − | (2) | AML | 113 | |
| NCT00086125 (2) | RRL | |||||
| PP242 | + | + | 117,118,157 | |||
| OSI-027 | + | − | 118,–120 | |||
| AZD-8050 | + | − | 118 | |||
| PI3K/mTOR | PI-103 | + | + | 76,126,127,158 | ||
| NVP-BEZ235 | + | + | 123,–125 | |||
| PI3K/PDK1 | BAG956 | + | + | 121 | ||
| PKB/PDK1/Flt3 | KP372-1 | + | − | 128 | ||
RRL indicates relapsed and refractory leukemia.
Research has also focused on the development of pharmacologic compounds that inhibit PKB. One such compound is perifosine, a synthetic alkylphosphocholine with oral bioavailability that inhibits PKB phosphorylation by competitive interaction with its PH domain and promotes degradation of PKB, mTOR, Raptor, Rictor, p70S6K, and 4E-BP1.101 It has been demonstrated that this compound induces apoptosis in multidrug-resistant human T-ALL cells and primary AML cells102,103 but does not affect normal CD34+ hematopoietic progenitor cells.103 The efficacy of perifosine in treatment of different types of leukemia is currently examined in several phase 2 clinical trials (NCT00391560 and NCT00873457). Phosphatidylinositol ether lipid analogs inhibit PKB activity in a similar manner compared with perifosine. Treatment of HL60 cells with phosphatidylinositol ether lipid analogs resulted in inhibition of proliferation and sensitization to chemotherapeutic agents in concentrations that did not affect proliferation of normal hematopoietic progenitors.104 Another specific PKB inhibitor (AKT-I-1/2 inhibitor) has been demonstrated to efficiently reduce colony formation in high-risk AML samples52 and induces apoptosis in primary CLL cells.105 The PKB inhibitor triciribine (API-2), a purine analog, has been demonstrated to interact with the PH domain of PKB and can thus prevent association of PKB with PI(3,4,5)P3. In T-ALL cell lines, API-2 has been demonstrated to induce cell cycle arrest and apoptosis.106 The safety of this inhibitor is currently examined in a phase 1 clinical trial in patients with advanced hematologic malignancies (#NCT00363454). Recently, a novel specific PKB inhibitor termed AiX has been demonstrated to preferentially induce apoptosis in CLL cells with a normal immunoglobulin status that correlates with poor clinical outcome.107
Rapamycin and its analogs RAD001 (everolimus), CCI-779 (temsirolimus), AP23573 (deforolimus), and RAD001108,109 inhibit the mTORC1 complex by association with FKBP-12, which abrogates the association of Raptor with mTOR.110 The efficacy of these compounds as therapeutic drugs has been examined in various preclinical and clinical studies for a wide range of malignancies. Although both rapamycin and its analog CCI-779 exhibit strong anti–tumor capacities in vitro,82,111,112 only a partial response was observed in clinical trials with rapamycin112 or its analog AP23573 in hematologic malignancies.113 The observed induction of PKB activity in AML blasts treated with rapamycin or AP23573 may explain the limited therapeutic effects of these compounds.114 Furthermore, experiments with PTEN-deficient mice revealed that withdrawal of rapamycin results in a rapid reinduction of leukemia and death in the majority of mice, which is probably because of failure to eliminate the leukemic stem cell population.115 In a subset of CLL patients, RAD-001 displays a modest antitumor activity.116 In addition, treatment of CLL patients with RAD-001 results in mobilization of malignant cells from the protective nodal masses into the peripheral blood.116 As an alternative, ATP-competitive mTOR inhibitors have recently been generated that inhibit the activity of both mTORC1 and mTORC2.117 Compared with rapamycin, the mTORC 1/2 inhibitor PP242 more efficiently reduced the development of leukemia in mice transplanted with primary ALL blasts or preleukemic thymocytes overexpressing PKB,117 while inducing less adverse effects on proliferation and function of normal lymphocytes.117,118 Similarly, OSI-027, another mTORC1/2 inhibitor,118 has been demonstrated to exhibit anti–leukemic effects in both Ph+ ALL and CML cells,119 and, compared with rapamycin, to efficiently suppress proliferation of AML cell lines.120
Dual inhibition of the PI3K/PKB pathway
Although the single-specificity inhibitors do affect the survival of leukemic cells, their effect in patients appears to be modest. The efficacy of combination therapy using multiple inhibitors directed against different intermediates of the PI3K signaling module is therefore also under investigation. Combined inhibition of mTOR and p110δ with RAD001 and IC87114, respectively, has for example been demonstrated to synergistically reduce proliferation of AML blasts.114 Similarly, combining the PI3K/PDK1 inhibitor BAG956 with RAD001 also resulted in a synergistic reduction in tumor volume in a mouse model transplanted with BCR-ABL–expressing cells.121 Finally, the efficacy of a novel combination regimen, including both perifosine and UCN-01 (NCT00301938), a PDK1 inhibitor that is known to induce apoptosis in AML cells in vitro,122 is currently examined in a phase 1 trial.
To further optimize inhibition of the PI3K signaling module, dual-specificity inhibitors have also been generated (Table 1). Recently, NVP-BEZ235, an orally bioavailable imidazoquinoline derivative that inhibits the activity of both PI3K and mTOR by binding to their ATP-binding pocket, has been identified.123 This compound significantly reduced proliferation and survival in both primary T-ALL124 and AML cells125 as well as leukemic cell lines124,125 without affecting the clonogenic capacity of normal hematopoietic progenitors.125 Another potent dual inhibitor for both class I PI3K isoforms and mTORC1 is PI-103, a synthetic small molecule of the pyridofuropyrimidine class. PI-103 has been demonstrated to reduce proliferation and survival of cells from T-ALL,126 AML,127 and CLL patients76 and appears to exhibit a stronger anti–leukemic activity compared with both rapamycin126 and the combination of RAD001 and IC87114.127 Importantly, although PI-103 reduces proliferation of normal hematopoietic progenitors, survival is not affected.127 A dual PI3K/PDK1 inhibitor called BAG956 has also recently been described. Although this compound can inhibit proliferation of BCR-ABL and FLT3-ITD–expressing cells, its anti–leukemic capacity is reduced compared with RAD001.121 In addition to these 3 dual-specificity inhibitors, KP372-1, a multiple kinase inhibitor capable of inhibiting PKB, PDK1, and FLT3, has been generated.128 It has been demonstrated that KP372-1 can induce apoptosis in primary AML cells and leukemic cell lines128 without affecting the survival of normal hematopoietic progenitors.128
Optimization of treatment strategies with combination therapy
Leukemogenesis involves aberrant regulation of various signal transduction pathways, including, but not limited to, the PI3K signaling module. Simultaneous targeting of multiple aberrantly regulated signal transduction pathways is therefore considered to be a promising therapeutic strategy (Tables 2 and 3). Histone deacetylase inhibitors have, for example, emerged as a promising class of anti–tumor agents. Although, in mouse models, the histone deacetylase inhibitor MS-275 only partially inhibited leukemic cells, combined administration of MS-275 and RAD001 potentiated the effect of both inhibitors individually both in vitro andin vivo.129 Synergistic negative effects on proliferation and survival of leukemic cell lines have also been observed after coadministration of histone deacetylase inhibitors and perisofine.130 Although proteosome inhibitors are considered to be a new class of therapeutic agents, treatment of pediatric and adult B-ALL patients with such an inhibitor (bortezomib) was not sufficient to induce a robust anti–tumor response.131 Experiments in leukemic cell lines and primary cells from B-ALL patients revealed that, whereas MG132, a proteosome inhibitor, and RAD001 alone only modestly reduce cell viability, combined inhibition of proteosomes and mTOR significantly enhanced cell death.132 In addition to proteosome inhibitors, the efficacy of specific tyrosine kinase inhibitors, including inhibitors of Flt3, Abl, and c-Kit, has been investigated in preclinical and clinical models. Combined inhibition of tyrosine kinases and the PI3K/PKB pathway resulted in a synergistically enhanced anti–leukemia effect in ALL121 and AML cells121 compared with the individual inhibitors. Phase 1 or 2 clinical trials have therefore been initiated to investigate the synergistic effects of combined inhibition of PI3K/PKB and Flt3 (NCT00819546) or c-Kit (NCT00762632). The occurrence of clinical resistance to STI-571 is a major problem for CML patients. Although the most common mechanisms of induction of resistance are mutations or alterations in the Bcr-Abl gene,133 a STI-571–induced compensatory PKB/mTor activation has also been described as a potential mechanism for the persistence of Bcr-Abl-positive cells in STI-571–treated patients.134-136 A combination of pharmacologic compounds inhibiting the PI3K/PKB pathway and STI-571 has been shown to be effective in cells from STI-571–resistant CML patients.119,121,137-142 Several phase 1 or 2 clinical trials have therefore been initiated to determine the effectiveness of such a combination therapy in STI-571–resistant CML patients (NCT01188889, NCT00093639, and NCT00101088).
Combination regimens with single specificity inhibitors
| Target . | Compound . | Combination regimens . | Effects . | Clinical trials (phase) . | Leukemia . | Reference(s) . | |
|---|---|---|---|---|---|---|---|
| In vitro . | In vivo . | ||||||
| PI3K | Wortmannin | ATRA (DA) | + | − | 159 | ||
| STI-571 (Bcr-Abl TKI) | + | − | 141 | ||||
| LY294002 | Apigenin (CK2 I) | + | − | 160 | |||
| ATRA (DA) | + | − | 159 | ||||
| STI-571 (Bcr-Abl TKI) | + | − | 141 | ||||
| Arsenic trioxide | + | − | 161 | ||||
| p110δ | IC87114 | VP16 (CT) | + | − | 145 | ||
| CAL-101 | Lenalidomide | + | − | 98 | |||
| CT and mAb CD20 | − | − | NCT01088048 (1) | CLL | |||
| Rituximab (mAb CD20) | − | − | NCT01203930 (2) | CLL | |||
| PDK1 | UCN-01 | Ara-C (CT) | + | − | (2) | AML | 146 |
| Cytarabine (CT) | − | − | NCT00004263 (1) | AML and MDS | |||
| Fludarabine (CT) | − | − | NCT00019838 (1) | RRL | |||
| OSU-03012 | STI-571 (Bcr-Abl TKI) | + | − | 140 | |||
| PP2A | Forskolin | Idarubicin/Ara-C | + | − | 162 | ||
| PKB | Perifosine | UCN-01 | − | − | NCT00301938 (1) | RRL | |
| HDAC I | + | − | 130 | ||||
| TRAIL (AI) | + | − | 163 | ||||
| Etoposide (CT | + | − | 103 | ||||
| PIA | CT | + | − | 104 | |||
| Triciribine | Cytarabine (CT) | + | − | 106 | |||
| MK2206 | Rituximab (mAb CD20) | − | − | NCT01369849 (1/2) | CLL | ||
| Arsenic trioxide | + | − | 161 | ||||
| mTOR | Rapamycin | UCN-01 | + | − | 122 | ||
| 3-BrOP (glycolysis I) | + | − | 164 | ||||
| STI-571 (Bcr-Abl TKI) | + | − | 139 | ||||
| Notch I | + | − | 165 | ||||
| Erlotinib (EGFR-TKI) | + | − | 166 | ||||
| Curcumin | + | − | 167 | ||||
| Dexamethasone | + | − | 168,169 | ||||
| Etoposide (CT) | + | + | 144 | ||||
| Decatibine (CT) | − | − | NCT00861874 (1) | AML | |||
| Aracytin (CT) | − | − | NCT00235560 (2) | AML | |||
| Methotrexate (CT) | + | + | NCT01162551 (2) | ALL and CLL | 111 | ||
| Anthracyclin (CT) | + | − | 170 | ||||
| Daunorubicin (CT) | + | − | 171 | ||||
| CT | + | − | NCT00776373 (1/2) | ALL and CML | 152 | ||
| + | − | NCT01184898 (1/2) | AML | 152 | |||
| + | − | NCT00780104 (1/2) | AML and CML | 152 | |||
| RAD001 | IC87114 | + | − | 114 | |||
| BAG956 | + | + | 121 | ||||
| Bortezomib (PI) | + | − | 132 | ||||
| MS-275 (HDAC I) | + | + | 129 | ||||
| PKC412 (Flt3 TKI) | − | − | NCT00819546 (1) | AML and MDS | |||
| Imatinib (Bcr-Abl TKI) | + | − | NCT00093639 (1/2) | CML | 138 | ||
| + | − | NCT01188889 (1/2) | CML | 138 | |||
| Nilotinib (c-Kit-TKI) | − | − | NCT00762632 (1/2) | AML | |||
| Alemtuzumab (mAb CD52) | − | − | NCT00935792 (1/2) | CLL | |||
| ATRA (DA) | + | + | 172 | ||||
| Ara-C (CT) | + | − | 132,149 | ||||
| Vincristine (CT) | + | + | 173 | ||||
| CT | − | − | NCT00544999 (1) | AML | |||
| − | − | NCT01154439 (1) | AML | ||||
| CCI-779 | Methotrexate (CT) | + | + | 111 | |||
| Clofarabine (CT) | − | − | NCT00775593 (2) | AML | |||
| STI-571 (Bcr-Abl TKI) | − | − | NCT00101088 (1) | CML | |||
| PP242 | Vincristine (CT) | + | − | 118 | |||
| OSI-027 | STI-571 (Bcr-Abl TKI) | + | − | 119 | |||
| GILZ | STI-571 (Bcr-Abl TKI) | + | − | 137 | |||
| Target . | Compound . | Combination regimens . | Effects . | Clinical trials (phase) . | Leukemia . | Reference(s) . | |
|---|---|---|---|---|---|---|---|
| In vitro . | In vivo . | ||||||
| PI3K | Wortmannin | ATRA (DA) | + | − | 159 | ||
| STI-571 (Bcr-Abl TKI) | + | − | 141 | ||||
| LY294002 | Apigenin (CK2 I) | + | − | 160 | |||
| ATRA (DA) | + | − | 159 | ||||
| STI-571 (Bcr-Abl TKI) | + | − | 141 | ||||
| Arsenic trioxide | + | − | 161 | ||||
| p110δ | IC87114 | VP16 (CT) | + | − | 145 | ||
| CAL-101 | Lenalidomide | + | − | 98 | |||
| CT and mAb CD20 | − | − | NCT01088048 (1) | CLL | |||
| Rituximab (mAb CD20) | − | − | NCT01203930 (2) | CLL | |||
| PDK1 | UCN-01 | Ara-C (CT) | + | − | (2) | AML | 146 |
| Cytarabine (CT) | − | − | NCT00004263 (1) | AML and MDS | |||
| Fludarabine (CT) | − | − | NCT00019838 (1) | RRL | |||
| OSU-03012 | STI-571 (Bcr-Abl TKI) | + | − | 140 | |||
| PP2A | Forskolin | Idarubicin/Ara-C | + | − | 162 | ||
| PKB | Perifosine | UCN-01 | − | − | NCT00301938 (1) | RRL | |
| HDAC I | + | − | 130 | ||||
| TRAIL (AI) | + | − | 163 | ||||
| Etoposide (CT | + | − | 103 | ||||
| PIA | CT | + | − | 104 | |||
| Triciribine | Cytarabine (CT) | + | − | 106 | |||
| MK2206 | Rituximab (mAb CD20) | − | − | NCT01369849 (1/2) | CLL | ||
| Arsenic trioxide | + | − | 161 | ||||
| mTOR | Rapamycin | UCN-01 | + | − | 122 | ||
| 3-BrOP (glycolysis I) | + | − | 164 | ||||
| STI-571 (Bcr-Abl TKI) | + | − | 139 | ||||
| Notch I | + | − | 165 | ||||
| Erlotinib (EGFR-TKI) | + | − | 166 | ||||
| Curcumin | + | − | 167 | ||||
| Dexamethasone | + | − | 168,169 | ||||
| Etoposide (CT) | + | + | 144 | ||||
| Decatibine (CT) | − | − | NCT00861874 (1) | AML | |||
| Aracytin (CT) | − | − | NCT00235560 (2) | AML | |||
| Methotrexate (CT) | + | + | NCT01162551 (2) | ALL and CLL | 111 | ||
| Anthracyclin (CT) | + | − | 170 | ||||
| Daunorubicin (CT) | + | − | 171 | ||||
| CT | + | − | NCT00776373 (1/2) | ALL and CML | 152 | ||
| + | − | NCT01184898 (1/2) | AML | 152 | |||
| + | − | NCT00780104 (1/2) | AML and CML | 152 | |||
| RAD001 | IC87114 | + | − | 114 | |||
| BAG956 | + | + | 121 | ||||
| Bortezomib (PI) | + | − | 132 | ||||
| MS-275 (HDAC I) | + | + | 129 | ||||
| PKC412 (Flt3 TKI) | − | − | NCT00819546 (1) | AML and MDS | |||
| Imatinib (Bcr-Abl TKI) | + | − | NCT00093639 (1/2) | CML | 138 | ||
| + | − | NCT01188889 (1/2) | CML | 138 | |||
| Nilotinib (c-Kit-TKI) | − | − | NCT00762632 (1/2) | AML | |||
| Alemtuzumab (mAb CD52) | − | − | NCT00935792 (1/2) | CLL | |||
| ATRA (DA) | + | + | 172 | ||||
| Ara-C (CT) | + | − | 132,149 | ||||
| Vincristine (CT) | + | + | 173 | ||||
| CT | − | − | NCT00544999 (1) | AML | |||
| − | − | NCT01154439 (1) | AML | ||||
| CCI-779 | Methotrexate (CT) | + | + | 111 | |||
| Clofarabine (CT) | − | − | NCT00775593 (2) | AML | |||
| STI-571 (Bcr-Abl TKI) | − | − | NCT00101088 (1) | CML | |||
| PP242 | Vincristine (CT) | + | − | 118 | |||
| OSI-027 | STI-571 (Bcr-Abl TKI) | + | − | 119 | |||
| GILZ | STI-571 (Bcr-Abl TKI) | + | − | 137 | |||
DA indicates differentiating agents; TKI, tyrosine kinase inhibitor; I, inhibitor; CT, chemotherapy; AI, apoptosis inducer; RRL, relapsed and refractory leukemia; and PI, proteasome inhibitor.
Combination regimens with dual specificity inhibitors
| Target . | Compound . | Combination regimens . | Effects . | Clinical trials (phase) . | References . | |
|---|---|---|---|---|---|---|
| In vitro . | In vivo . | |||||
| PI3K/mTOR | PI-103 | Nutlin-3 (MDM2-I) | + | − | 158 | |
| STI-571 (Bcr-Abl-TKI) | + | − | 142 | |||
| Arsenic disulfide | + | − | 174 | |||
| Vincristine (CT) | + | − | 126 | |||
| Fludarabine (CT) | + | − | 76 | |||
| PI3K/mTOR | NVP-BEZ235 | CT | + | − | 124 | |
| PI3K/PDK1 | BAG956 | STI-571 (Bcr-Abl-TKI) | + | + | 121 | |
| Rapamycin/RAD-001 | + | − | 121 | |||
| PKC412 (Flt3 TKI) | + | + | 121 | |||
| Target . | Compound . | Combination regimens . | Effects . | Clinical trials (phase) . | References . | |
|---|---|---|---|---|---|---|
| In vitro . | In vivo . | |||||
| PI3K/mTOR | PI-103 | Nutlin-3 (MDM2-I) | + | − | 158 | |
| STI-571 (Bcr-Abl-TKI) | + | − | 142 | |||
| Arsenic disulfide | + | − | 174 | |||
| Vincristine (CT) | + | − | 126 | |||
| Fludarabine (CT) | + | − | 76 | |||
| PI3K/mTOR | NVP-BEZ235 | CT | + | − | 124 | |
| PI3K/PDK1 | BAG956 | STI-571 (Bcr-Abl-TKI) | + | + | 121 | |
| Rapamycin/RAD-001 | + | − | 121 | |||
| PKC412 (Flt3 TKI) | + | + | 121 | |||
I indicates inhibitor; TKI, tyrosine kinase inhibitor; and CT, chemotherapy.
Chemotherapy has been shown to be effective in a subset of patients. However, incomplete remission and the development of a refractory disease have been observed in many patients with acute leukemia.143 To optimize treatment, chemotherapy could potentially be combined with specific pharmacologic inhibitors (Table 2). Coadministration of mTOR inhibitors with different types of chemotherapeutic drugs, including etoposide, Ara-C, cytarabine, and dexamethasone, has, for example, been demonstrated to induce synergistic anti–leukemia effects in cells from AML patients144 and ALL patients.111,132 Several phase 1 or 2 clinical trials have therefore been initiated to investigate and optimize the synergistic effect of mTOR inhibitors and chemotherapeutic drugs in patients (NCT00544999, NCT01184898, NCT00780104, NCT01162551 and NCT00776373, NCT00861874, NCT00235560, and NCT01154439) In addition, synergistic effects have been observed in AML cells after coadministration of chemotherapeutic agents with IC87114,145 UCN-01,146 or triciribine.106 Similar results were obtained in T-ALL cells when combining the dual-specificity inhibitors PI-103 and NVP-BEZ235 with chemotherapy.124,126 Finally, inhibition of the activity of p110δ with CAL-101 was shown to be sufficient to abrogate lenalidomide-induced activation of primary CCL cells.98 In addition to the clinical trials focusing on CAL-101 alone, the efficacy of a combination regimen with both CAL-101 and lenalidomide is therefore also under investigation in phase 1 or 2 clinical trials (NCT01203930 and NCT01088048).
Future perspectives
During the last 2 decades, it has become clear that the PI3K/PKB signal transduction pathway plays an important role in both normal and malignant hematopoiesis. As discussed in “Derequlated P13K/PKB signaling in malignant hematopoiesis,” aberrant regulation of this signaling module has been observed in a large group of acute leukemias. Although mutations in PI3K, PKB, or the upstream regulators PTEN and SHIP1 have been detected in cells from patients with leukemia, these mutations appear to be rare. These mutations can therefore not account for the large incidence of constitutive activation of PI3K in patients with leukemia. In contrast, several oncogenic fusion proteins and mutated tyrosine kinase receptors have been demonstrated to induce hematologic malignancies by constitutive activation of the PI3K/PKB signaling module. Because PI3K is frequently activated in leukemia and activation of this molecule is thought to correlate with poor prognosis and drug resistance, it is considered to be a promising target for therapy. A high number of pharmacologic inhibitors directed against both individual and multiple components of this pathway have already been developed to improve therapy. Although the single-specificity inhibitors do affect the survival of leukemic cells, their effect in patients appears to be modest. This can be explained by both inhibitor-induced abrogation of negative feedback loops and alternative mechanisms of activation of downstream effectors of PI3K/PKB. Although further research is required to examine the safety and efficacy of the dual-specificity inhibitors, these compounds appear to possess more promising anti–leukemic activities compared with single-specificity inhibitors. In addition, to further optimize therapeutic regimens, research has focused on coadministration of inhibitors of the PI3K signaling module with either classic chemotherapy or inhibitors directed against other signal transduction molecules. The in vitro studies and mouse transplantation experiments described herein strongly suggest that both strategies could indeed be used to improve current therapeutic regimens in specific patient groups.
Inhibition of aberrantly regulated intracellular signal transduction pathways provides an important means to improve therapeutic regimens for patients with leukemia. Because the PI3K/PKB signaling pathway appears to be highly deregulated in a large number of patients with leukemia, this pathway is considered to be a promising target for therapy. Compared with targeting individual components of the PI3K/PKB signaling module alone, either abrogating this pathway at multiple levels using dual-specificity inhibitors or combining pathway specific inhibitors with classic regimens appears to be a more effective therapeutic strategy for patients with leukemia. Further research is therefore warranted to examine the safety and efficacy of these regimens in leukemia patients.
Acknowledgments
R.P. was supported by KiKa (Children Cancer Free grant).
The authors apologize that, because of space restrictions, some references to original papers may not have been included in this review.
Authorship
Contribution: R.P. and M.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Miranda Buitenhuis, Department of Hematology, Erasmus MC, Dr Molewaterplein 50, Faculty Building Office H-Ee1330F, 3015 GE Rotterdam, The Netherlands; e-mail: m.buitenhuis@erasmusmc.nl.