Abstract
Lenalidomide plus dexamethasone is effective in the treatment of multiple myeloma (MM) but is associated with an increased risk of venous thromboembolism (VTE). This prospective, open-label, randomized substudy of a phase 3 trial compared the efficacy and safety of thromboprophylaxis with low-dose aspirin (ASA) or low-molecular-weight heparin (LMWH) in patients with newly diagnosed MM, treated with lenalidomide and low-dose dexamethasone induction and melphalan-prednisone-lenalidomide consolidation. Overall, 342 patients who did not have clinical indications or contraindications to antiplatelet or anticoagulant therapy were randomly assigned to receive ASA 100 mg/d (n = 176) or LMWH enoxaparin 40 mg/d (n = 166). The incidence of VTE was 2.27% in the ASA group and 1.20% in the LMWH group. Compared with LMWH, the absolute difference in the proportion of VTE was 1.07% (95% confidence interval, −1.69-3.83; P = .452) in the ASA group. Pulmonary embolism was observed in 1.70% of patients in the ASA group and none in the LMWH group. No arterial thrombosis, acute cardiovascular events, or sudden deaths were reported. No major hemorrhagic complications were reported. In previously untreated patients with MM receiving lenalidomide with a low thromboembolic risk, ASA could be an effective and less-expensive alternative to LMWH thromboprophylaxis. This study was registered at www.clinicaltrials.gov as #NCT00551928.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1093.
Disclosures
Alessandra Larocca has received honoraria from Janssen-Cilag. Federica Cavallo has received honoraria from Celgene and Janssen-Cilag. Sara Bringhen has received honoraria from Celgene, Janssen-Cilag, Novartis, and Merck. Francesco Di Raimondo has received honoraria from and served on the speakers' bureau of Celgene and Janssen-Cilag. Anna Falanga has served on the scientific advisory board of Pfizer and Sanofi-Aventis. Maide Cavalli has received honoraria from Janssen-Cilag, Celgene, and Novartis and served on the speakers' bureau of Janssen-Cilag and Millennium Pharmaceuticals. Francesca Patriarca has served on the advisory board of Celgene, Janssen-Cilag, and Schering-Plough. Michele Cavo has received honoraria from and served on the advisory board of Janssen-Cilag, Millennium Pharmaceuticals, Celgene, and Novartis. Maria Teresa Petrucci has received honoraria from Celgene and Janssen-Cilag. Tommaso Caravita Di Toritto has received honoraria and consultancy from Celgene. Mario Boccadoro has received research support from and has served as a consultant and on the scientific advisory board of Celgene and Janssen-Cilag. Antonio Palumbo has received honoraria from Celgene, Janssen-Cilag, Merck, and Amgen and served on the advisory committee of Celgene and Janssen-Cilag. The Associate Editor A. Keith Stewart has served as an advisor for Onyx Pharmaceuticals Inc, Novartis Pharmaceuticals Corporation, Celgene Corporation, and Millennium Pharmaceuticals Inc. He has also received grants for clinical research from Millennium Pharmaceuticals Inc. The remaining authors and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Compare incidence of VTE in newly diagnosed patients treated with lenalidomide for multiple myeloma who received thromboprophylaxis with low-dose ASA or LMWH, based on a large, prospective, randomized trial.
Compare incidence of pulmonary embolism and other adverse events in newly diagnosed patients treated with lenalidomide for multiple myeloma who received thromboprophylaxis with low-dose ASA or LMWH, based on a large, prospective, randomized trial.
Descrive strengths and limitations of this large, prospective, randomized trial of thromboprophylaxis in newly diagnosed patients treated with lenalidomide for multiple myeloma.
Release date: January 26, 2012; Expiration date: January 26, 2013
Introduction
The annual incidence of venous thromboembolism (VTE), which encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE), is > 1 per 1000 in the general population,1 but it increases to > 7% in patients with cancer.2 The risk of cancer-associated thrombosis depends on the type of cancer the patient has and is particularly high in patients with hematologic malignancies, especially multiple myeloma (MM). The baseline risk of developing VTE in patients with cancer is increased 28-fold compared with persons without cancer.3 The introduction of the oral immunomodulatory drugs, thalidomide and lenalidomide, has improved myeloma outcomes, but these agents are also associated with higher rates of VTE.4,5 Individual risk factors for VTE associated with thalidomide and lenalidomide therapy include advanced age, a history of VTE, an indwelling central venous catheter, comorbid conditions (eg, infections, diabetes, cardiac disease), current or recent immobilization, recent surgery, and inherited thrombophilic abnormalities.6
Lenalidomide in combination with high-dose dexamethasone is an effective and well-tolerated treatment for patients with relapsed and refractory MM7,8 and is also highly effective in patients with newly diagnosed MM (NDMM).9 However, lenalidomide in combination with high-dose dexamethasone is associated with VTE rates of 26%-67% in patients with NDMM.9,10 In contrast, the risk of VTE is significantly reduced to 12% with low-dose dexamethasone.9 In the relapsed/refractory setting, the risk of VTE in patients treated with lenalidomide plus high-dose dexamethasone is 11%-15%.7,8
Because of the increasing use of immunomodulatory agent-based treatment combinations, the prevention of VTE has become an important consideration during myeloma treatment.9-13 On the basis of the available data, the current American Society of Clinical Oncology guidelines recommend thromboprophylaxis with low-molecular-weight heparin (LMWH) or adjusted-dose warfarin in patients receiving combination regimens, including lenalidomide.14 The use of LMWH is limited by factors such as a high cost of treatment, lower patient compliance because of the need for self-injection, and renal impairment. The International Myeloma Working Group recommends aspirin (ASA) prophylaxis for patients with 1 or no VTE risk factors, and LMWH for those with > 1 risk factors for VTE.6 The comparative efficacy of different single-agent antithrombotic regimens in myeloma is not established. However, a recent randomized, open-label study in patients with untreated NDMM reported that ASA and fixed low-dose warfarin were as effective as LMWH in reducing the incidence of serious thrombotic events associated with thalidomide regimens.15
The aim of this substudy of a phase 3, multicenter, randomized controlled clinical trial was to compare the efficacy and safety of ASA and LMWH, in preventing VTE in patients with MM treated with lenalidomide as first-line therapy.
Methods
Study design and treatment
This open-label, multicenter, randomized trial was conducted at 62 centers in Italy and Israel from November 2007 to June 2009, as a substudy of the phase 3 randomized controlled trial RV-MM-PI209. This study compared the efficacy and safety of the consolidation regimen melphalan-prednisone-lenalidomide (MPR) with the standard high-dose melphalan 200 mg/m2 (Mel200) regimen followed by tandem stem cell transplantation in patients with NDMM. The aim of the substudy was to compare the effectiveness and safety of ASA and LMWH as antithrombotic prophylaxis in patients receiving lenalidomide-based induction and consolidation therapy.
The study was approved by the institutional review board at each of the participating centers. All patients gave written informed consent before entering the study, which was performed in accordance with the Declaration of Helsinki. The study was designed by the investigators, who were also responsible for the data collection; A.L., F.C., and A.E. analyzed the data; and all authors had access to the primary clinical trial data.
Patient populations and randomization
Previously untreated patients with NDMM, aged between 18 and 65 years, enrolled in the phase 3 trial were assessed for eligibility to be enrolled in the substudy. Eligible patients had no history of DVT or arterial thromboembolic events within the past 12 months, no clear indication or contraindication for antiplatelet or anticoagulant therapy, had no active bleeding, and were not considered to be at high risk of bleeding. Eligible patients were randomly assigned to receive ASA 100 mg/d orally, or LMWH enoxaparin 40 mg/d subcutaneously, using a simple randomization sequence run by a central computer, which generated an automated assignment procedure that was concealed from the investigators in each study center.
All patients enrolled in the substudy received induction with lenalidomide plus low-dose dexamethasone (Rd) treatment comprising four 28-day cycles of lenalidomide (25 mg/d orally for 21 days) in combination with dexamethasone (40 mg/d orally on days 1, 8, 15, and 22), followed by cyclophosphamide (4 g/m2) for stem cell mobilization and collection before entering the consolidation phase with either MPR or Mel200. The MPR consolidation phase comprised six 28-day cycles of lenalidomide 10 mg/d for 21 days, melphalan 0.18 mg/kg for 4 days, and prednisone 2 mg/kg for 4 days.
Study interventions
Prophylaxis was administered during the 4 cycles of Rd therapy and the 6 cycles of MPR consolidation. Patients who were assigned to the Mel200 consolidation arm stopped thromboprophylaxis at this point. Antithrombotic prophylaxis was discontinued in any patient who developed DVT, PE, arterial thrombosis, or any acute cardiovascular or bleeding event or patient who had a platelet count of ≤ 50 000/μL. Patients attended clinic study visits every 2 weeks during the first 2 cycles of Rd or MPR, then every 4 weeks for the last 2 cycles of Rd and the last 4 cycles of MPR to assess the effectiveness and safety of treatment. Subsequently, patients attended visits at the physician's discretion, and the incidence of thromboembolism in the absence of prophylaxis was also evaluated.
Outcome measures
The primary end point was a composite measure defined as the proportion of patients developing a first episode of objectively confirmed symptomatic DVT, PE, arterial thrombosis, any acute cardiovascular event (acute myocardial infarction or stroke), or sudden, otherwise unexplained, death (presumed to be related to PE, acute myocardial infarction, or stroke) in the first 6 months after random assignment. Secondary end points included the incidence of major and minor bleeding events and any other complications related to thromboprophylaxis.
All adverse events were assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (Version 3.0).16 Major bleeding was defined as fatal bleeding, symptomatic bleeding in a crucial area or organ, or bleeding that caused a reduction in hemoglobin concentration of ≥ 2 g/dL or that necessitated transfusion of ≥ 2 units of whole blood or red blood cells.17 The incidence of minor bleeding comprised all bleeding events that did not meet the criteria for major bleeding.
Data on age, sex, performance status according to Karnofsky score, standard prognostic parameters (serum levels of creatinine, β2-microglobulin, and serum albumin), and comorbidities (diabetes, cardiovascular disease, orthopedic surgery, dyslipidemia, prior thromboembolism, and concomitant recombinant human erythropoietin administration) were collected.
Statistical analysis
The main planned comparison was ASA versus LMWH during the first 12 months after randomization. The statistical power and the minimum effect size detectable in this substudy was determined according to the prevalence of patients without a clear indication to anticoagulant or antiplatelet therapy in the sample size of the main phase 3 trial (RV-MM-PI209). Overall, 402 patients treated with lenalidomide-containing regimens were randomly assigned, with a 1:1 allocation ratio between the two prophylaxis regimens. The expected rate of thromboembolic events in patients with NDMM treated with lenalidomide-containing regimens was ∼ 12%-67%.9,10 Of the 402 patients enrolled in the RV-MM-PI209 study, 60 patients were not eligible for this substudy. The sample size of342 patients reaches statistical power, ranging from 47% to 80% to detect an absolute difference of 7%-11%, respectively, between the groups, with α of 0.05 (2-tailed), assuming a value of 10% for the composite primary end point in the LMWH group.
The statistical test used to compare the difference between proportions was the 2-sided z test, with pooled variance. To compare the incidence of the composite primary end point in the 2 intervention arms through to follow-up, taking into account the competing risk of death because of any other cause, the cumulative incidence (adjusted for competing risks) was compared between groups with the use of the Gray test.18 All efficacy and safety analyses were performed according to the intention-to-treat principle and included all randomly assigned patients who received ≥ 1 dose of the study drug. Times of observation were censored on November 30, 2010. Data were analyzed in December 2010 with the use of SAS Version 8.2 software with SPSS 18.0.2 (SPSS Inc) packages and R Version 2.5.0 software (package cmprsk; open source).
Results
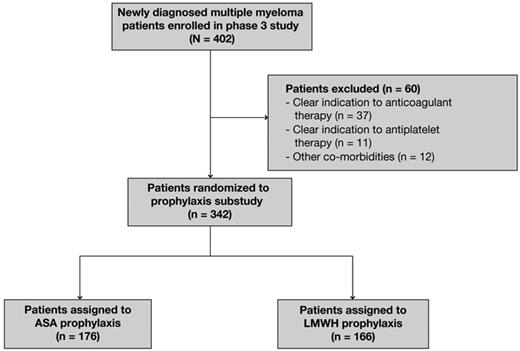
A total of 402 patients aged ≤ 65 years with NDMM were enrolled in the study and received induction with Rd. Of the 402 patients assessed for eligibility, 342 patients were enrolled in this substudy and were included in the efficacy and safety analyses; 176 were randomly assigned to receive ASA and 166 were randomly assigned to receive LMWH (Figure 1). Sixty patients were excluded from the substudy because of a clear indication to anticoagulant therapy (n = 37) or antiplatelet therapy (n = 11) or other comorbidities (n = 12). The main reasons for exclusion were recent orthopedic surgery or vertebroplasty, immobilization, allergy to ASA, concomitant thromboembolism at diagnosis, concomitant disseminated intravascular coagulation, inherited thrombophilic abnormalities, previous history of coronary ischemic disease or angioplasty, atrial fibrillation, and glucose-6-phosphate-dehydrogenase deficiency.
Flow diagram of patients enrolled in the trial. ASA indicates aspirin; and LMWH, low-molecular-weight heparin.
Flow diagram of patients enrolled in the trial. ASA indicates aspirin; and LMWH, low-molecular-weight heparin.
Baseline patient characteristics are summarized in Table 1. The median age of patients at diagnosis was similar between the 2 prophylaxis groups (57 years in the ASA group and 58 years in the LMWH group). Overall, 49% of the patients in the ASA group and 60% in the LMWH group were men (P = .058). According to International Staging System criteria, 43% and 54% of patients in the ASA and LMWH groups, respectively, had stage I myeloma; for stage II disease these percentages were 31% and 27%, respectively, and for stage III the percentages were 26% and 19%, respectively (P = .082). All enrolled patients in the study had symptomatic disease requiring antimyeloma therapy. The 2 prophylaxis groups were well balanced in terms of patient characteristics and the number of comorbid conditions, as assessed by medical history. Overall, 48% of patients in both the ASA and LMWH arms were assigned to consolidation with MPR and 52% to Mel200. Four patients (2%) in the ASA and 2 patients (1%) in the LMWH group had ≥ 2 risk factors for VTE.
Baseline characteristics of study population
| Characteristic . | ASA (n = 176) . | LMWH (n = 166) . |
|---|---|---|
| Median age at diagnosis, y (IQR) | 57 (51-61) | 58 (52-62) |
| Age, y, n (%) | ||
| 31-40 | 4 (2) | 2 (1) |
| 41-50 | 35 (20) | 29 (17) |
| 51-60 | 74 (42) | 69 (42) |
| ≥ 60 | 63 (36) | 66 (40) |
| Sex, n (%) | ||
| Male | 87 (49) | 99 (60) |
| Multiple myeloma treatment, n (%) | ||
| Rd | 176 (100) | 166 (100) |
| MPR | 85 (48) | 80 (48) |
| Mel200 | 91 (52) | 86 (52) |
| International Staging System stage, n (%) | ||
| I | 75 (43) | 90 (54) |
| II | 55 (31) | 45 (27) |
| III | 46 (26) | 31 (19) |
| Median creatinine level, mg/dL (IQR) | 0.9 (0.76-1.13) | 0.9 (0.8-1.1) |
| Median glycemia level, mg/dL (IQR) | 93 (84-103) | 94 (88-105) |
| Karnofsky performance status score, n (%) | ||
| ≤ 70 | 27 (15) | 23 (14) |
| Data missing | 0 | 1 (1) |
| Risk factors, n (%)* | ||
| Diabetes | 2 (1) | 2 (1) |
| Cardiovascular disease | 12 (7) | 6 (4) |
| Hypertension | 12 (7) | 6 (4) |
| Coronary arterial disease | 0 | 1 (1) |
| Dyslipidemia | 3 (2) | 0 |
| Orthopedic surgery | 0 | 0 |
| Prior thromboembolism | 0 | 0 |
| Inherited coagulopathies | 0 | 0 |
| Recombinant human erythropoietin | 30 (17) | 28 (17) |
| ≥ 2 risk factors | 4 (2) | 2 (1) |
| Characteristic . | ASA (n = 176) . | LMWH (n = 166) . |
|---|---|---|
| Median age at diagnosis, y (IQR) | 57 (51-61) | 58 (52-62) |
| Age, y, n (%) | ||
| 31-40 | 4 (2) | 2 (1) |
| 41-50 | 35 (20) | 29 (17) |
| 51-60 | 74 (42) | 69 (42) |
| ≥ 60 | 63 (36) | 66 (40) |
| Sex, n (%) | ||
| Male | 87 (49) | 99 (60) |
| Multiple myeloma treatment, n (%) | ||
| Rd | 176 (100) | 166 (100) |
| MPR | 85 (48) | 80 (48) |
| Mel200 | 91 (52) | 86 (52) |
| International Staging System stage, n (%) | ||
| I | 75 (43) | 90 (54) |
| II | 55 (31) | 45 (27) |
| III | 46 (26) | 31 (19) |
| Median creatinine level, mg/dL (IQR) | 0.9 (0.76-1.13) | 0.9 (0.8-1.1) |
| Median glycemia level, mg/dL (IQR) | 93 (84-103) | 94 (88-105) |
| Karnofsky performance status score, n (%) | ||
| ≤ 70 | 27 (15) | 23 (14) |
| Data missing | 0 | 1 (1) |
| Risk factors, n (%)* | ||
| Diabetes | 2 (1) | 2 (1) |
| Cardiovascular disease | 12 (7) | 6 (4) |
| Hypertension | 12 (7) | 6 (4) |
| Coronary arterial disease | 0 | 1 (1) |
| Dyslipidemia | 3 (2) | 0 |
| Orthopedic surgery | 0 | 0 |
| Prior thromboembolism | 0 | 0 |
| Inherited coagulopathies | 0 | 0 |
| Recombinant human erythropoietin | 30 (17) | 28 (17) |
| ≥ 2 risk factors | 4 (2) | 2 (1) |
ASA indicates aspirin; LMWH, low-molecular-weight heparin; IQR, interquartile range; Rd, lenalidomide and low-dose dexamethasone; MPR, melphalan, prednisone plus lenalidomide; and Mel200, melphalan as conditioning regimen for autologous stem cell transplantation.
Risk factors include diabetes, cardiovascular disease, orthopedic surgery during the past 3 months, dyslipidemia, prior thromboembolism in the medical history, inherited coagulopathies, concomitant recombinant human erythropoietin administration.
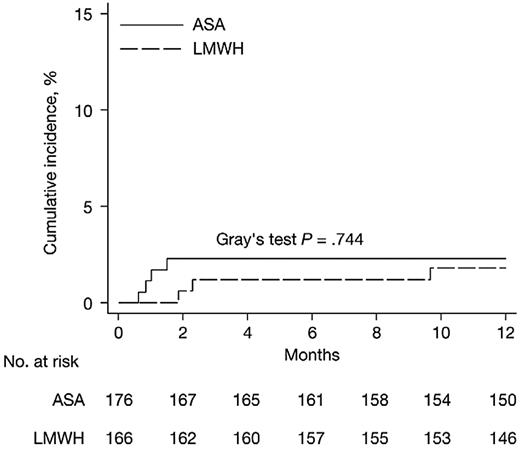
Overall, 88% of patients in the substudy finished the planned Rd induction therapy; the median follow-up period was 20 months. During the first 6 months from randomization, the incidence of grade 3/4 DVT and PE was 2.27% (4 of 176) in patients receiving ASA and 1.20% (2 of 166) in patients receiving LMWH prophylaxis (P = .452; Table 2). Symptomatic PE occurred in 1.70% (3 of 176) patients in the ASA group; there were no reports of PE in the LMWH group. The 3 patients who experienced PE had all received concomitant recombinant human erythropoietin during Rd induction. Four patients treated with ASA experienced a superficial thrombophlebitis; no cases of superficial phlebitis were reported in the LMWH group. No arterial thrombosis, acute cardiovascular events, or sudden deaths were reported in either the ASA or LMWH groups (Table 2). The absolute difference in the composite primary end point between the ASA and LMWH groups during the first 6 months of follow-up was +1.07% (95% confidence interval [CI], −1.69-3.83; P = .452), the absolute difference in the incidence of DVT was −0.07% (95% CI, −2.35-2.21; P = .953), and the absolute difference in the incidence of PE was 1.70% (95% CI, −0.21-3.62; P = .091) for the ASA versus LMWH groups (Table 2). The cumulative proportions of thromboembolic events, acute cardiovascular events, and sudden deaths adjusted for competing risks at 12 months were 2.3 (95% CI, 0.1-4.5) in the ASA group and 1.8 (95% CI, 0.0-3.9) in the LMWH group (Figure 2).
The incidence and absolute risk difference of the composite primary end point during the first 6 months of follow-up for aspirin (ASA) compared with low-molecular-weight heparin (LMWH) prophylaxis
| Event . | ASA (n = 176) . | LMWH (n = 166) . | Absolute difference, % . | 95% CI . | P . |
|---|---|---|---|---|---|
| Efficacy analysis, n (%) | |||||
| Composite primary end point* | 4 (2.27) | 2 (1.20) | 1.07 | −1.69 to 3.83 | .452 |
| Any grade 3/4 thromboembolic event, n (%) | 4 (2.27) | 2 (1.20) | 1.07 | −1.69 to 3.83 | .452 |
| Deep vein thrombosis | 2 (1.14) | 2 (1.20) | −0.07 | −2.35 to 2.21 | .953 |
| Pulmonary embolism | 3 (1.70) | 0 | 1.70 | −0.21 to 3.62 | .091 |
| Arterial thrombosis | 0 | 0 | |||
| Acute cardiovascular events | 0 | 0 | |||
| Sudden deaths | 0 | 0 | |||
| Safety analysis, n (%) | |||||
| Major bleeding | 0 | 0 | |||
| Minor bleeding | 1 (0.60) | −0.60 | −1.78 to 0.57 | .302 | |
| Gastrointestinal bleeding | 0 | 1 (0.60) |
| Event . | ASA (n = 176) . | LMWH (n = 166) . | Absolute difference, % . | 95% CI . | P . |
|---|---|---|---|---|---|
| Efficacy analysis, n (%) | |||||
| Composite primary end point* | 4 (2.27) | 2 (1.20) | 1.07 | −1.69 to 3.83 | .452 |
| Any grade 3/4 thromboembolic event, n (%) | 4 (2.27) | 2 (1.20) | 1.07 | −1.69 to 3.83 | .452 |
| Deep vein thrombosis | 2 (1.14) | 2 (1.20) | −0.07 | −2.35 to 2.21 | .953 |
| Pulmonary embolism | 3 (1.70) | 0 | 1.70 | −0.21 to 3.62 | .091 |
| Arterial thrombosis | 0 | 0 | |||
| Acute cardiovascular events | 0 | 0 | |||
| Sudden deaths | 0 | 0 | |||
| Safety analysis, n (%) | |||||
| Major bleeding | 0 | 0 | |||
| Minor bleeding | 1 (0.60) | −0.60 | −1.78 to 0.57 | .302 | |
| Gastrointestinal bleeding | 0 | 1 (0.60) |
The composite primary end point was the first episode of any objectively confirmed symptomatic deep vein thrombosis, pulmonary embolism, arterial thrombosis, acute cardiovascular event (acute myocardial infarction or stroke), or sudden otherwise unexplained death (presumed to be related to pulmonary embolism, acute myocardial infarction, or stroke).
Cumulative incidence of the primary composite end point, adjusted for competing risks (death of any cause) by treatment group. The end of the follow-up period was defined as 12 months because no further venous thromboembolism events occurred after this time. ASA indicates aspirin; and LMWH, low-molecular-weight heparin.
Cumulative incidence of the primary composite end point, adjusted for competing risks (death of any cause) by treatment group. The end of the follow-up period was defined as 12 months because no further venous thromboembolism events occurred after this time. ASA indicates aspirin; and LMWH, low-molecular-weight heparin.
The characteristics of patients who experienced grade 3/4 thromboembolic events during the substudy are detailed in Table 3. The median age at onset of VTE was 57 years. Most thrombotic events occurred early during Rd treatment, with a median time to onset of 1.3 months. The median times to onset in the 2 prophylaxis arms were 0.95 months in the ASA group and 2.13 months in the LMWH group (Figure 2). One patient in the LMWH group and no patients in the ASA group experienced a thromboembolic event during consolidation treatment with MPR.
Characteristics of patients who experienced grade 3/4 thromboembolic events during prophylaxis
| Patient . | Characteristics at multiple myeloma diagnosis . | Characteristics at VTE onset . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of prophylaxis . | Age, y . | Sex . | ISS stage . | KPS score . | Risk factors . | VTE . | Onset after enrollment, mo . | Treatment . | |
| 1 | ASA | 57 | Male | II | 70 | rHuEpo | PE | 1.57 | Rd |
| 2 | ASA | 55 | Female | III | 70 | rHuEpo | PE | 0.63 | Rd |
| 3 | LMWH | 58 | Female | III | 80 | None | DVT | 1.93 | Rd |
| 4 | ASA | 57 | Female | I | 70 | rHuEpo | PE | 0.87 | Rd |
| 5 | ASA | 61 | Female | III | 90 | None | DVT | 1.03 | Rd |
| 6 | LMWH | 52 | Male | I | 100 | None | DVT | 2.33 | Rd |
| 7 | LMWH | 56 | Male | I | 100 | None | DVT | 9.8 | MPR |
| Patient . | Characteristics at multiple myeloma diagnosis . | Characteristics at VTE onset . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of prophylaxis . | Age, y . | Sex . | ISS stage . | KPS score . | Risk factors . | VTE . | Onset after enrollment, mo . | Treatment . | |
| 1 | ASA | 57 | Male | II | 70 | rHuEpo | PE | 1.57 | Rd |
| 2 | ASA | 55 | Female | III | 70 | rHuEpo | PE | 0.63 | Rd |
| 3 | LMWH | 58 | Female | III | 80 | None | DVT | 1.93 | Rd |
| 4 | ASA | 57 | Female | I | 70 | rHuEpo | PE | 0.87 | Rd |
| 5 | ASA | 61 | Female | III | 90 | None | DVT | 1.03 | Rd |
| 6 | LMWH | 52 | Male | I | 100 | None | DVT | 2.33 | Rd |
| 7 | LMWH | 56 | Male | I | 100 | None | DVT | 9.8 | MPR |
VTE indicates venous thromboembolism; ISS, International Staging System; KPS, Karnofsky performance status; ASA, aspirin; rHuEpo, recombinant human erythropoietin; PE, pulmonary embolism; Rd, lenalidomide and low-dose dexamethasone; LMWH, low-molecular-weight heparin; DVT, deep vein thrombosis; and MPR, melphalan, prednisone plus lenalidomide.
The small number of VTE events precluded meaningful analysis of the relationship between baseline characteristics and VTE occurrence.
No major bleeding complications were detected during thromboprophylaxis with either ASA or LMWH (Table 2). Only 1 event of gastrointestinal minor bleeding, which had a complete and spontaneous resolution, was reported in the LMWH group.
The median duration of prophylaxis was 3.6 and 3.5 months in the ASA and LMWH groups, respectively. Twelve patients (6.8%) in the ASA group and 5 patients (3%) in the LMWH group discontinued prophylaxis prematurely, mainly because of progressive disease during Rd induction (6 patients in the ASA group and 3 patients in the LMWH group) and adverse events (3 patients in the ASA group and 1 patient in the LMWH group).
Discussion
This is the first large prospective randomized study that compared ASA with LMWH thromboprophylaxis in patients with NDMM treated with an Rd induction regimen. Our findings show that in patients with NDMM without a high individual risk of VTE, and without a clear indication to anticoagulant or antiplatelet therapy, prophylaxis with either ASA or LMWH during lenalidomide-based induction treatment is associated with a low VTE incidence. The incidence of VTE was 2.27% in the ASA group and 1.20% in the LMWH group. No acute cardiovascular events and sudden deaths were reported. In our study most thromboembolic events occurred early during the first months of lenalidomide therapy. Treatment was delayed in patients experiencing a VTE, but no discontinuations or treatment interruptions because of thromboembolic events were reported. No evidence of early or late deaths because of thromboembolism was seen during lenalidomide treatment.
In patients receiving Rd at diagnosis, and for whom antithrombotic prophylaxis was not mandatory, the incidence of VTE was 12%.9 The VTE rate observed during Rd induction and MPR consolidation in our study was much lower than previously reported in patients with NDMM. Because of the fixed sample size of the main trial RV-MM-PI209, it was not possible to increase the power of the substudy.
Prevention of VTE is an important consideration during myeloma treatment because of the increasing use of immunomodulatory agent-based treatment combinations. The development of thrombotic events is a potentially life-threatening complication that may lead to treatment discontinuations, increases in patient morbidity, and increased health care costs. A recent population-based study found that the rate of hospitalization because of VTE in a large cohort of patients with cancer was more than double that in a cohort of the general population (1.8% vs 0.8%, respectively).19 The risk of VTE in patients with cancer was increased 8-fold during the first year after cancer diagnosis, declining thereafter but remaining twice as high as in persons without cancer.19
In our trial, no significant differences in VTE incidence and safety profile were found between patients treated with ASA and LMWH, but our study includes patients with myeloma who had a standard risk of VTE, in other words in those whose risk was not increased because of the presence of individual factors such as advanced age, inherited thrombophilic abnormalities, recent surgery, or a history of VTE. Three PE events were observed in patients receiving ASA, whereas no PE was detected in the LMWH group. These findings are comparable with the results of another study performed by our group on thromboprophylaxis in patients with NDMM treated with thalidomide-based regimens. We reported that ASA and low-dose warfarin were as effective as LMWH in reducing the incidence of VTE, acute cardiovascular events, and sudden deaths, with the exception of elderly patients for whom warfarin showed less efficacy than LMWH.15 Further clinical studies are required to evaluate the role of initial short-term treatment with LMWH, followed by long-term ASA to reduce the risk of PE, regardless of baseline individual thrombotic risk.
In the present study, the concomitant use of erythropoietin is the only risk factor reported in patients who experienced PE. A previous trial has also reported an increased incidence of VTE in patients with cancer treated with erythropoiesis-stimulating agents, compared with patients who did not receive this support (7% vs 1%, respectively).20 In our trial, no direct correlation between the use of erythropoietin and the incidence of VTE can be suggested because of the limited number of events reported, but further evaluations are warranted.
Although arterial thrombotic events are rare and little is known about the underlying mechanisms, they are increasingly recognized as a complication of hematologic cancer. A recent report, including population-based data from 18 627 patients with MM, found an increased risk of both arterial thrombosis and VTE than with matched controls.21 Case reports of patients treated with thalidomide also suggest that arterial thrombosis may be an adverse effect of this drug. In one study, including 195 previously untreated young patients, the incidence of arterial thrombosis was 5.6%, independent of induction treatment and was strongly associated with well-known risk factors, including hypertension and smoking.22 Therefore, we included both venous and arterial events as end points in the present study. However, no arterial thromboses were reported, probably because of the exclusion of patients receiving long-term antithrombotic treatment. Furthermore, our study population comprised young patients, with a relatively low incidence of risk factors for arterial thrombosis, such as hypertension.
Patients with MM are at higher risk of developing thrombocytopenia because of disease infiltration of the bone marrow and as an adverse effect of cytotoxic therapy. This may increase the risk of bleeding associated with thromboprophylaxis in myeloma. In the present study no major bleeding events were reported in association with low-dose ASA, indicating that this may be a safe prophylactic option in patients with NDMM. Other studies on thromboprophylaxis in patients with cancer have, however, reported conflicting results about the risk of bleeding associated with ASA prophylaxis,23-25 and further evaluation is needed. Patients with myeloma require regular and continued monitoring of platelet counts during MM treatment and during thromboprophylaxis. If thrombocytopenia develops, ASA should be withheld when the platelet count falls to 50 000/μL and the dose of the LMWH reduced. A 50% reduction in LMWH dose is recommended for patients with a platelet count < 50 000/μL, with LMWH treatment discontinued if the platelet count is < 20 000/μL.26
A main strength of the study is the prospective and randomized design that enabled us to analyze a well-defined group of previously untreated patients with myeloma; almost all the randomly assigned patients were included in the efficacy and safety analyses. However, the study had several limitations. First, only a small number of VTE events were detected, which limited our analysis of the associations between patient risk factors and the development of VTE. However, thrombotic events did not appear to be associated with well-known risk factors for VTE, including infections, diabetes, cardiac disease, immobilization, surgery, and inherited thrombophilia. Patient selection was the second significant limitation of our study because only patients with standard risk of VTE aged < 65 years were included. Patients with high risk of VTE, such as those with a history of thromboembolism, severe cardiovascular disease, uncontrolled diabetes, infections, immobilization, or surgery, were excluded from the substudy because they had a clear indication or contraindication to a specific anticoagulant or antiplatelet therapy. Finally, a placebo comparison was not possible for ethical reasons because of the known high VTE risk associated with lenalidomide and dexamethasone treatment regimens.
The 2008 International Myeloma Working Group consensus statement on the prevention of thrombosis associated with thalidomide and lenalidomide recommends specific thromboprophylaxis strategies according to the type of therapy and the individual risk of patients, taking into account bleeding and clotting risks.6 Our results support the use of VTE risk stratification-based prophylactic strategies and may suggest a new consensus for antithrombotic prophylaxis. With the exception of clear indications or contraindications, the use of ASA and LMWH could be optimized by adopting the following approach. After an individualized thrombotic risk assessment, prophylaxis with LMWH should be mandatory in patients at high risk of VTE during induction therapy with lenalidomide. However, ASA could be a safe and effective option during induction therapy with lenalidomide for standard-risk patients with no or only 1 VTE risk factor and for prophylaxis during long-term treatment, such as consolidation and maintenance with lenalidomide, to reduce the occurrence of late thromboembolic events.
The potential benefit and the safety profile of next-generation oral anticoagulants, including direct thrombin inhibitors (dabigatran etexilate), factor Xa inhibitors (rivaroxaban, apixaban) and defibrotide, could be assessed in patients with MM receiving immunomodulatory drugs. In a phase 1/2 study, only 1 VTE event was detected with defibrotide in combination with melphalan, prednisone, and thalidomide, in patients with relapsed/refractory MM without further thromboprophylaxis.27 Defibrotide exerts a spectrum of pleiotropic effects on endothelium, hemostasis, and stroma, which makes it a potential protective agent against thromboembolism.
In conclusion, this study shows that the risk of VTE in patients with NDMM receiving lenalidomide is low independent of the prophylaxis used. LMWH showed a clear benefit in reducing lenalidomide-associated VTE complications, whereas ASA was not as effective in preventing PE. ASA could be considered an alternative option to LMWH for prophylaxis in untreated patients with NDMM, with low individual risk of thrombosis, with the advantages of oral administration, safe use in comorbid conditions (eg, renal insufficiency), no need for regular coagulation monitoring, being inexpensive, and the possibility of long-term administration. However, the small number of events detected makes it difficult to draw general conclusions, and further evaluations to optimize patient care are still needed.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who agreed to participate in this study; the nurses, in particular Elena Ponticelli and Maria Marcella Lionetti; the data managers, Maria Josè Fornaro, Jessica Mastrovito, Giulia Lupparelli, and Debora Caldarazzo; and the editorial assistant, Giorgio Schirripa. They thank Excerpta Medica for linguistic improvement, formatting of the manuscript, and artwork.
The study RV-MM-PI209 was supported by Fondazione Neoplasie Sangue Onlus. Celgene supplied free lenalidomide for the study RV-MM-PI209.
Celgene had no role in the study design, data analysis, data interpretation, or writing of the report.
Authorship
Contribution: M.B. and A.P. designed the research; A.L. and F.C. collected, analyzed, and interpreted data; A.E. performed statistical analysis; A.L., F.C., M.B., and A.P. wrote the manuscript; S.B., F.D.R., A.F., M.C., A. Stanevsky, P.C., S.P., F.P., M.C., J.P., L.C., A. M. Carella, A. M. Cafro, A. Siniscalchi, C.C., M.T.P., D.B.Y., E.B., T.C.D.T., and A.N. enrolled patients; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: A.L. has received honoraria from Janssen-Cilag; F.C. has received honoraria from Celgene and Janssen-Cilag; S.B. has received honoraria from Celgene, Janssen-Cilag, Novartis, and Merck; F.D.R. has received honoraria from and served on the speakers' bureau of Celgene and Janssen-Cilag; A.F. has served on the scientific advisory board of Pfizer and Sanofi-Aventis; M.C. has received honoraria from Janssen-Cilag, Celgene, and Novartis and served on the speakers' bureau of Janssen-Cilag and Millennium Pharmaceuticals; F.P. has served on the advisory board of Celgene, Janssen-Cilag, and Schering-Plough; M.C. has received honoraria from and served on the advisory board of Janssen-Cilag, Millennium Pharmaceuticals, Celgene, and Novartis; M.T.P. has received honoraria from Celgene and Janssen-Cilag; T.C.D.T. has received honoraria and consultancy from Celgene; M.B. has received research support from and has served as a consultant and on the scientific advisory board of Celgene and Janssen-Cilag; and A.P. has received honoraria from Celgene, Janssen-Cilag, Merck, and Amgen and served on the advisory committee of Celgene and Janssen-Cilag. The remaining authors declare no competing financial interests.
Correspondence: Antonio Palumbo, Myeloma Unit, Division of Hematology, University of Torino, AOU S. Giovanni Battista, Torino, 10126, Italy; e-mail: appalumbo@yahoo.com.