In this issue of Blood, Kuo et al have used recombinant lectin-like domain of thrombomodulin domain 1 (TMD1) to demonstrate the action of lectin-like domain in blocking Lewis Y antigen (LeY)–mediated angiogenesis and control of tumor growth.1
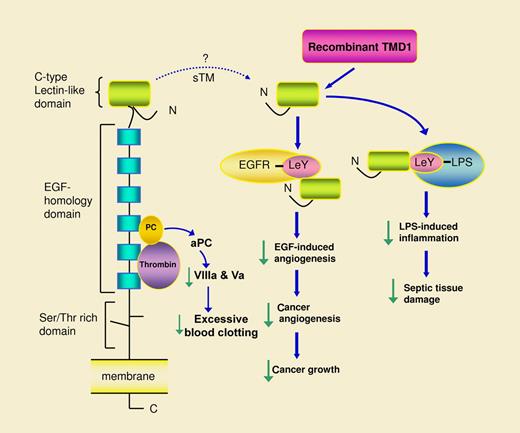
Thrombomodulin (TM) anchors to vascular endothelial cell membrane with a single transmembranous domain.2,3 It has a large extracellular region that comprises a C-type lectin-like domain at the N-terminus, an epidermal growth factor (EGF)–homology domain that contains 6 EGF-like structures, and a Ser/Thr-rich domain linked to the membrane (see figure). Thrombin and protein C bind to the EGF-homology domain where thrombin cleaves protein C to generate activated protein C (aPC).2 aPC is released into the blood where together with protein S it degrades coagulation factors VIIIa and Va and thereby prevents excessive blood clotting. aPC also possesses anti-inflammatory actions and is effective in controlling inflammatory tissue damage caused by sepsis. Biochemical mechanisms and physiologic roles of TM in coagulation and inflammation have been well characterized.2 However, biochemical characterization has been focused on the thrombin and protein C binding sites at the EGF-homology domain. Little is known about the biologic activity and physiologic role of the C-type lectin-like domain. Kuo and colleagues have generated recombinant TMD1 and used it to probe the biologic activity of the lectin-like domain. They previously reported in Blood that TMD1 binds specifically to LeY and through its binding to Lewis Y epitope on lipopolysaccharide (LPS), it suppresses LPS-induced inflammatory responses.4 Here, Kuo et al provide evidence that recombinant TMD1 controls endothelial cell migration and tube formation through binding to LeY clustered at the endothelial cell membrane ruffles and protrusions.1 Their results show that LeY mediates angiogenesis and TMD1 or LeY antibodies abrogate the angiogenic effect. Epidermal growth factor receptor (EGFR) is expressed on endothelial cell surface. It contains LeY that may be involved in angiogenesis. Investigations in vivo show that TMD1 blocks in vivo angiogenesis induced by EGF and suppresses tumor growth by inhibiting tumor angiogenesis. Taken together, the results indicate that the lectin-like domain of TM is a receptor for LeY that blocks angiogenesis and attenuates inflammatory tissue damage by binding and neutralizing LeY.
Recombinant thrombomodulin domain 1 (TMD1, equivalent to lectin-like domain) binds Lewis Y antigen (LeY)–containing proteins such as epidermal growth factor receptor (EGFR) via which it neutralizes the angiogenic action of EGF and thereby suppresses tumor angiogenesis and growth. TMD1 was previously shown to bind LeY-containing lipopolysaccharide (LPS) and attenuate LPS-induced inflammation and tissue damage. The dotted lime depicts possible release of the lectin-like domain into circulating blood as a component of soluble thrombomodulin (sTM). Soluble lectin-like domain may act in a manner similar to TMD1 and may serve as a sensor of LeY for control of LeY-induced pathophysiologic processes.
Recombinant thrombomodulin domain 1 (TMD1, equivalent to lectin-like domain) binds Lewis Y antigen (LeY)–containing proteins such as epidermal growth factor receptor (EGFR) via which it neutralizes the angiogenic action of EGF and thereby suppresses tumor angiogenesis and growth. TMD1 was previously shown to bind LeY-containing lipopolysaccharide (LPS) and attenuate LPS-induced inflammation and tissue damage. The dotted lime depicts possible release of the lectin-like domain into circulating blood as a component of soluble thrombomodulin (sTM). Soluble lectin-like domain may act in a manner similar to TMD1 and may serve as a sensor of LeY for control of LeY-induced pathophysiologic processes.
LeY refers to a tetrasaccharide moiety (Fucose α 1,2 Galatose β 1,4(Fucose α 1,3) N-acetylglucosamine), which is attached to proteins or lipids. LeY is related to blood group Lewis a and b. However, its biologic activities are distinct from the Lewis blood group. LeY has been shown to play an important role in inflammation and tissue damage induced by LPS of diverse microorganisms including Helicobactor pylori.5 LeY has recently been implicated in cancer cell proliferation and tumor growth in vivo that were considered to be mediated via EGF signaling.6,7 Findings from the report by Kuo et al provide new insights into the role of LeY-containing EGF receptors in angiogenesis, and based on the effect of recombinant TMD1 on suppressing tumor growth, the results imply that LeY promotes tumor growth in part via inducing angiogenesis.
Even with the information provided by Kuo et al, the physiologic role of TM in angiogenesis is far from clear. Because the intact TM possesses little activity on endothelial tube formation and angiogenesis, it is unlikely that the membrane-anchored TM is directly involved in control of angiogenesis. The reason why intact TM is devoid of antiangiogenic action is unclear. It may be speculated that the lectin-like domain is structurally blocked in the intact TM. Recombinant TMD1 represents a soluble form of TM fragments that are released from the structural block and the lectin domain is free to interact with LeY. This raises an intriguing question: are there lectin-like domains in circulating blood that may function as a LeY sensor to control angiogenesis and LPS-induced inflammation and tissue damage? There is no clear answer. However, it is well recognized that normal plasma contains TM fragments (soluble TM, or sTM) with molecular masses ranging from 105 kDa to 28 kDa.8,9 The high molecular weight fragments were shown to possess thrombin binding activity. It is unknown whether the soluble TM possesses LeY binding activity. Judging from the molecular masses of sTM, it is possible that the low molecular weight fragments at ∼ 30 kDa may contain the lectin-like domains. Soluble TM is shedded from membrane TM in healthy humans by mechanisms not entirely understood. Population-based epidemiologic study has shown that plasma sTM of healthy subjects is inversely associated with coronary heart disease,10 suggesting that sTM may play a protective role against cardiovascular injury. Soluble lectin-like domain may contribute to the protective effect. Further studies are needed to clarify this important issue.
Lectin-like domain of TM is like a hidden treasure. You do not see it in intact TM, and it shines when it is separated from TM. Recombinant TMD1 paves the way for elucidating the important roles of lectin-like domain in health and disease and serves as a lead for new drug development.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal