Abstract
Recent work has established that heterozygous germline GATA2 mutations predispose carriers to familial myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML), “MonoMAC” syndrome, and DCML deficiency. Here, we describe a previously unreported MDS family carrying a missense GATA2 mutation (p.Thr354Met), one patient with MDS/AML carrying a frameshift GATA2 mutation (p.Leu332Thrfs*53), another with MDS harboring a GATA2 splice site mutation, and 3 patients exhibiting MDS or MDS/AML who have large deletions encompassing the GATA2 locus. Intriguingly, 2 MDS/AML or “MonoMAC” syndrome patients with GATA2 deletions and one with a frameshift mutation also have primary lymphedema. Primary lymphedema occurs as a result of aberrations in the development and/or function of lymphatic vessels, spurring us to investigate whether GATA2 plays a role in the lymphatic vasculature. We demonstrate here that GATA2 protein is present at high levels in lymphatic vessel valves and that GATA2 controls the expression of genes important for programming lymphatic valve development. Our data expand the phenotypes associated with germline GATA2 mutations to include predisposition to primary lymphedema and suggest that complete haploinsufficiency or loss of function of GATA2, rather than missense mutations, is the key predisposing factor for lymphedema onset. Moreover, we reveal a crucial role for GATA2 in lymphatic vascular development.
Introduction
GATA2 is a zinc finger transcription factor that plays crucial roles in hematopoiesis1,2 and urogenital3 and neural development.4,5 We and others have recently identified heterozygous GATA2 germline mutations, both inherited and de novo, in patients with myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML),6 “MonoMAC” syndrome of monocytopenia with predisposition to nontubercular mycobacterial infection,7 and the syndrome of dendritic cell, monocyte, and B and NK lymphoid deficiency (“DCML deficiency”).8 Five missense and 2 small in-frame deletion GATA2 mutations described in these syndromes lie within the conserved second zinc finger (ZF2) of GATA2 and have been demonstrated (c.1061C > T, p.Thr354Met and c.1063_1065delACA, p.Thr355del),6 or predicted, to result in loss of GATA2 transcriptional activity because of loss of GATA2 binding to its consensus WGATAR DNA binding motif. However, ZF2 of GATA2 is also involved in protein-protein interactions important for the formation of transcriptional complexes, and the ZF2 mutations p.Thr354Met and p.Thr355del retain dominant negative activity in some functional assays.6 Of particular interest, mutations in 3 ZF2 residues (p.Thr354, p.Arg396, and p.Arg398) have been found in multiple independent studies.6-8 Previous work has established that recurrent mutations in oncogenes are a hallmark of dominant negative mutations,9 suggesting that, in addition to p.Thr354Met, p.Arg396Trp/Gln and p.Arg398Trp may act in a dominant negative manner. Four frameshift mutations in GATA2 and 2 large intragenic GATA2 deletions have also been described in the MonoMAC/DCML syndrome. These mutations most probably result in complete loss of function (LOF) causing GATA2 haploinsufficiency.7,8
Multiple lines of evidence have implicated GATA2 in vascular development. GATA2 has been demonstrated to bind and regulate the promoter activity of endothelial genes, including PECAM1,10 Kdr (encoding VEGFR-2),11 ANGPT2,12 and EMCN,13 and GFP knocked into the Gata2 locus is expressed in endothelial cells of the heart, blood vessels, and lymphatic vessels during mouse embryogenesis.14 A definitive role for GATA2 during vascular development has, however, remained enigmatic, as vascular defects are not obvious in Gata2−/− mice before their death at approximately embryonic day 10 (E10),1 and very few studies have documented GATA2 protein expression in the vasculature in vivo.
Lymphatic vessels are crucial for tissue fluid homeostasis, immune cell trafficking, and absorption of dietary fats.15 Primary, hereditary lymphedema is a chronic, debilitating condition caused by the failure of lymphatic vessels to develop and/or function to full capacity, resulting in accumulation of fluid and protein in affected tissues. To date, mutations in only a handful of genes have been associated with human hereditary lymphedema. Mutations in genes encoding the tyrosine kinase receptor VEGFR-3,16 the transcription factor SOX18,17 and the collagen and calcium-binding EGF-domain-1 protein CCBE118 are causative of Milroy disease, hypotricosis-lymphedema-telangiectasia, and Hennekam syndrome, respectively. Each of these genes controls crucial processes in lymphatic vascular growth and development. Mutations in the transcription factor FOXC2 underlie lymphedema-distichiasis19 and disrupt lymph flow because of the failure of valves to form in collecting lymphatic vessels. Recent work has revealed that mutations in the gap junction protein Connexin 47, encoded by GJC2, are associated with human hereditary lymphedema, possibly also because of disrupting lymphatic flow.20
Here we report on 7 new patients and 3 previously described patients7,21 with germline GATA2 mutations, including complete and partial gene deletions, who display a range of clinical features consistent with MDS/AML and MonoMAC/DCML deficiency.7,8 Two patients with GATA2 deletions and one with a GATA2 frameshift mutation also presented with primary lymphedema, which led us to investigate the role of GATA2 in the lymphatic vasculature. We demonstrate that Gata2 protein is present in lymphatic vessels of embryonic and adult mice and that Gata2 levels are particularly high in the leaflets of lymphatic vascular valves. Furthermore, knockdown of Gata2 in primary lymphatic endothelial cells (LECs) abrogates the expression of genes, including Prox1, Foxc2, Angpt2, and Itga9, which are important for valve development. Our data demonstrate that, in addition to hematologic disorders, GATA2 mutations may result in primary lymphedema and reveal a previously unrecognized role for GATA2 in lymphatic vascular development.
Methods
Human ethics
Patients gave informed consent in accordance with the Declaration of Helsinki for institutional review board–approved protocols at the National Institutes of Health, Royal Adelaide Hospital (100702), and the University of Washington (Seattle, WA).
Cytogenetic testing using array CGH
Patient 6 was analyzed using an oligonucleotide-based, 105K-feature, whole genome array (SignatureChip Oligo Solution Version 1.1, custom designed by Signature Genomics, and manufactured by Agilent Technologies), according to previously described methods.22 Patient 4 was analyzed using an oligonucleotide-based, 135K-feature, whole genome array (SignatureChip Oligo Solution Version 2.0, custom designed by Signature Genomics, manufactured by Roche NimbleGen), according to previously described methods.23
Whole exome capture/GATA2 sequencing
Patients 2 and 3 were subjected to exome sequencing, as previously described.24 Briefly, paired end libraries with 300-bp inserts were hybridized to the SeqCap EZ Human Exome Library Version 2.0 (NimbleGen). Sequencing was performed with 2 × 101-bp reads using SBS Version 3 on a HiSeq (Illumina) to a median read depth of 132×, with 93.3% of the exome covered by a minimum of 10 reads. Exome variants were filtered against common polymorphisms within dbSNP132 and the Exome Sequencing Project (http://snp.gs.washington.edu/EVS; accessed August 5, 2011). Rare and private variants were classified by predicted function to include all missense, nonsense, frameshift, or splice-site alleles. GATA2 was amplified and sequenced as previously described,6,7 using DNA from either buccal samples (parents of patient 7), fibroblasts taken from a 3-mm skin biopsy (patient 7) or bone marrow (patient 10).
Animal studies
Experiments using mice were performed using C57Bl/6 mice and were approved and conducted in accordance with the SA Pathology/Central Health Network Animal Ethics Committee and Australian National Health and Medical Research Council guidelines.
Isolation and culture of primary LECs and BECs
Embryonic mouse LECs and blood endothelial cells (BECs) were isolated from E16.5 dermis and cultured as previously described.25
Transfection of primary LECs
All cells were transfected using LipofectamineTM 2000 (Invitrogen) according to the manufacturer's instructions and Gata2 Stealth RNAi siRNA (Invitrogen Oligo ID MSS204584, Target Accession NM_008090.4). Freshly isolated LECs were seeded on fibronectin coated 24-well dishes at a density of 5 × 104 cells/well, cultured overnight, and then transfected with 20 pmol siRNA (33nM final concentration). Twenty-four hours later, cells were transfected with a further 20 pmol siRNA. Cells were harvested 48 hours later and used for RNA and protein analyses.
RNA analysis
Total RNA was isolated from freshly isolated and transfected cells using TRIzol reagent (Invitrogen), according to the manufacturer's instructions. RNA quality was assessed using a Bioanalyser (Agilent Technologies); all samples achieved an RNA Integrity Number score more than 9.5. For investigation of mRNA expression levels, total RNA was reverse-transcribed using Superscript III Reverse Transcriptase (Invitrogen) with a mixture of oligo dT and random hexamer primers. Primers used for real-time RT-PCR analysis are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). PCR was performed with RT2 Real-Time SYBR Green/Rox PCR master mix (SA Biosciences) and analyzed on a Corbett Rotor-Gene 6000. Data were normalized to the housekeeping gene Actb as previously described.26 Quantification of mature miR-451 in primary embryonic mouse LECs and BECs was performed using a TaqMan MicroRNA Assay (Applied Biosystems ID #001141) and normalized to snoRNA202 (Applied Biosystems ID #001232).
Western blotting
Protein was isolated using TPER reagent with Protease Inhibitors (both from Thermo Scientific) or TRIzol reagent (Invitrogen). After RNA extraction with TRIzol, protein was recovered from the organic phase according to the manufacturer's instructions. For analysis of protein levels in transfected cells, equal amounts of protein isolated from 3 separate transfections were pooled and electrophoresed on 8% SDS-PAGE gels before being transferred to polyvinylidene difluoride (PerkinElmer). Western blots were performed according to standard protocols using anti–GATA-2 (Santa Cruz Biotechnology, CG2-96), anti-Prox1 (AngioBio), and anti–β-actin (Sigma-Aldrich) antibodies, followed by anti–rabbit AP (GE Healthcare) and anti–mouse Cy5 (GE Healthcare). Signal was detected using ECF Western blot substrate (GE Healthcare), and blots were scanned on a Typhoon Imager (GE Healthcare). Densitometry was performed with ImageQuant TL Version 7.0 software (GE Healthcare).
Immunostaining
Primary LECs isolated from E16.5 embryonic skin were cultured on fibronectin-coated chamber slides for 3 days, fixed with 4% phosphate-buffered paraformaldehyde (PFA) and immunostained as previously described.8 E16.5 embryos or whole adult ears were fixed in 4% PFA overnight or for 4 hours, respectively. All steps were carried out at room temperature unless stated otherwise. Tissues were permeabilized in PBS with 0.3% (volume/volume) TritonX-100 (PBST) for 15 minutes before blocking in PBST containing 1% (weight/volume) BSA overnight at 4°C. Tissues were then incubated with anti-Prox1 (R&D Systems), anti-CD31 (BioLegend), and anti–GATA-2 (Santa Cruz Biotechnology, H-116) or anti-CD45 (BD Biosciences PharMingen) antibodies overnight at 4°C. After extensive washing in PBST, samples were incubated with AlexaFluor-488, -555, and -647 conjugated secondary antibodies (Invitrogen) overnight at 4°C. Samples were again washed extensively in PBST, postfixed using 4% PFA, dehydrated via a graded methanol series, and then cleared and mounted in benzyl alcohol:benzyl benzoate (1:2). Images were captured using a Bio-Rad Radiance 2100 confocal microscope (Bio-Rad) equipped with 3 lasers (488-nm Argon ion, 543-nm Green HeNe, and 637-nm Red Diode), attached to an Olympus IX70 inverted microscope (Olympus), using a UApo/340 20×/0.7 Wα/0.17 H2O objective. Image acquisition was performed at room temperature using Lasersharp 2000 software (Version 5.2, Bio-Rad). Adobe Photoshop CS5 Version 12.0 (Adobe) was used for subsequent image processing.
Results
Phenotypes of patients with GATA2 mutations
Here we report on 10 patients: 3 (patients 1-3, Table 1) from a newly identified family with MDS carrying the recurrent c.1061C > T (p.Thr354Met) mutation in the GATA2 gene, 3 unrelated persons with MDS/AML who have large heterozygous de novo germline deletions resulting in removal of the entire GATA2 gene, 2 patients from a previously described MonoMAC pedigree with a deletion including the first 2 coding exons of GATA2, probably a complete LOF allele, and thus haploinsufficient, one with a previously unreported GATA2 frameshift mutation (p.Leu332Thrfs*53), and one with a previously unreported GATA2 splice-site mutation (Table 1). In the family with MDS (supplemental Figure 1), the p.Thr354Met missense mutation was found using whole exome sequencing. Of the 3 affected persons for which samples were available (patients 1-3), all were diagnosed with MDS, and 2 displayed chronic infections (patients 1 and 3). This is consistent with clinical features of other reported familial or de novo p.Thr354Met-carrying persons diagnosed with MonoMAC syndrome/DCML deficiency.7,8 In our 3 previously reported families with p.Thr354Met, MDS/AML was also partially penetrant, with apparently unaffected persons remaining healthy into their 60s.6 Furthermore, only 1 of a total of 32 persons in our previous study with MDS, AML, or nonspecific leukemia carrying the p.Thr354Met mutation showed chronic mycobacterial infection characteristic of the MonoMAC syndrome.7,27 MDS was also a feature of patient 10, who we found to have a novel splice donor site mutation in GATA2 that we predict to affect correct exon 4/exon 5 splicing. In addition to MDS and similarly to patients 8 and 9 diagnosed with MonoMAC syndrome, patient 10 displayed warts.
Genetic and clinical details of persons with GATA2 mutations
| Patient ID . | No. of patients . | Familial or de novo . | GATA2 mutation . | Genomic, cDNA, and protein change . | GATA2 function . | Overall phenotype . | Lymphedema (age onset, y) . | Hematologic abnormalities (age onset, y) with acquired chromosomal anomalies . | Immunodeficiency/infections . | Publications . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 (FHCRC-97) | 1 female (daughter) | Familial | p.Thr354Met | chr3:g.128 200 744G > A GATA2 c.1061C > T (p.Thr354Met) | DB(−)* | M/A, ID | None | MDS with 5q-clone, aplastic anemia (10) | Recurrent febrile illness, parainfluenza, mycoplasma | Current study |
| Patient 2 (FHCRC-84) | 1 female (daughter) | Familial | p.Thr354Met | chr3:g.128 200 744G > A GATA2 c.1061C > T (p.Thr354Met) | DB(−)* | M/A | None | MDS (14) with 5%-6% monosomy 7, thrombocytopenia (14) | No | Current study |
| Patient 3 (FHCRC-95) | 1 female (mother) | Familial | p.Thr354Met | chr3:g.128 200 744G > A GATA2 c.1061C > T (p.Thr354Met) | DB(−)* | M/A, ID | None | MDS (44) with trisomy 8, leukopenia, anemia, thrombocytopenia (15) | Recurrent infections (pneumonias) as a child | Current study |
| Patient 4 (GC54819) | 1 male | De novo | Contiguous gene deletion encompassing GATA2 gene | chr3:g.127 966 423-136 853 218 (minimum) chr3:g.127 927 712-136 889 323 (maximum) (8.9 Mb 3q21.3-q22.3 deletion) | Null | M/A, ID, DF, NS | None | MDS (dysplastic megakaryopoiesis), leukopenia, anemia, thrombocytopenia, dysplastic megakaryopoiesis | Infections associated with pancytopenia | Current study |
| Patient 5 (French girl) | 1 female | De novo | Contiguous gene deletion encompassing GATA2 gene | 6.9 Mb interstitial 3q21.1-q21.3 deletion (genes ADCY5 - TRH) (∼ chr3:g.123 000 000-129 700 000) | Null | M/A, ID, DF, NS | None | MDS (11) with monosomy 7 on 7/22 metaphases, pancytopenia (11) | Gingivitis and staphylococcal skin infections | Current study, Callier21 |
| Patient 6 (GC42542) | 1 male | De novo | Contiguous gene deletion encompassing GATA2 gene | chr3:g.120 247 726–128 319 968 (minimum) chr3:g.120 154 188-128 324 987 (maximum) (8.2 Mb 3q13.33-q21.3 deletion) | Null | L, M/A, ID, DF, NS | Lymphedema (6.5; bilateral) | MDS/AML (16/29) with trisomy 21 | Recurrent pneumonia and ear infections, H influenza meningitis | Current study, Wildin (submitted) |
| Patient 7 | 1 male | De novo | p.Leu332Thrfs*53 | chr3:g.128 202 727-128 202 728ins GGTC GATA2 c.992_993insGACC (p.Leu332Thrfs*53) | LOF ZF(−) | L, M/A | Lymphedema (birth; bilateral) | Pancytopenia (14) with monosomy 7, MDS (18) with monosomy 7 and trisomy 8, AML (18) | No | Current study |
| Patient 8, kindred 13.I.2 | 1 female (mother) | Familial | p.Met1del290 | chr3:g.128 204 043-128 211 896 GATA2 c.1-200_871 + 527del 2033 bp (p.Met1del290) | Null | L, ID | Lymphedema (unilateral left) | Monocytopenia and B-cell, NK-cell, and T-cell lymphocytopenia | Warts (adulthood), no history of infections disseminated MAC, fungal infections, warts, recurrent pneumonias | Current study, Vinh,27 Hsu7 |
| Patient 9, kindred 13.II.1 | 1 male (son) | Familial | p.Met1del290 | chr3:g.128 204 043-128 211 896 GATA2 c.1-200_871 + 527del 2033 bp (p.Met1del290) | Null | M/A | None | MDS (33) with monosomy 7 on all metaphases, trisomy 8, pancytopenia (33) | Current study, Vinh,27 Hsu7 | |
| Patient 10 | 1 male | De novo | p.? | chr3:g.128 202 701A>C GATA2 c.1017 + 2T>G (p.?) | LOF ZF(−) | M/A, ID | None | MDS (19), monocytopenia (19) | Warts, severe acne | Current study |
| Patient ID . | No. of patients . | Familial or de novo . | GATA2 mutation . | Genomic, cDNA, and protein change . | GATA2 function . | Overall phenotype . | Lymphedema (age onset, y) . | Hematologic abnormalities (age onset, y) with acquired chromosomal anomalies . | Immunodeficiency/infections . | Publications . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 (FHCRC-97) | 1 female (daughter) | Familial | p.Thr354Met | chr3:g.128 200 744G > A GATA2 c.1061C > T (p.Thr354Met) | DB(−)* | M/A, ID | None | MDS with 5q-clone, aplastic anemia (10) | Recurrent febrile illness, parainfluenza, mycoplasma | Current study |
| Patient 2 (FHCRC-84) | 1 female (daughter) | Familial | p.Thr354Met | chr3:g.128 200 744G > A GATA2 c.1061C > T (p.Thr354Met) | DB(−)* | M/A | None | MDS (14) with 5%-6% monosomy 7, thrombocytopenia (14) | No | Current study |
| Patient 3 (FHCRC-95) | 1 female (mother) | Familial | p.Thr354Met | chr3:g.128 200 744G > A GATA2 c.1061C > T (p.Thr354Met) | DB(−)* | M/A, ID | None | MDS (44) with trisomy 8, leukopenia, anemia, thrombocytopenia (15) | Recurrent infections (pneumonias) as a child | Current study |
| Patient 4 (GC54819) | 1 male | De novo | Contiguous gene deletion encompassing GATA2 gene | chr3:g.127 966 423-136 853 218 (minimum) chr3:g.127 927 712-136 889 323 (maximum) (8.9 Mb 3q21.3-q22.3 deletion) | Null | M/A, ID, DF, NS | None | MDS (dysplastic megakaryopoiesis), leukopenia, anemia, thrombocytopenia, dysplastic megakaryopoiesis | Infections associated with pancytopenia | Current study |
| Patient 5 (French girl) | 1 female | De novo | Contiguous gene deletion encompassing GATA2 gene | 6.9 Mb interstitial 3q21.1-q21.3 deletion (genes ADCY5 - TRH) (∼ chr3:g.123 000 000-129 700 000) | Null | M/A, ID, DF, NS | None | MDS (11) with monosomy 7 on 7/22 metaphases, pancytopenia (11) | Gingivitis and staphylococcal skin infections | Current study, Callier21 |
| Patient 6 (GC42542) | 1 male | De novo | Contiguous gene deletion encompassing GATA2 gene | chr3:g.120 247 726–128 319 968 (minimum) chr3:g.120 154 188-128 324 987 (maximum) (8.2 Mb 3q13.33-q21.3 deletion) | Null | L, M/A, ID, DF, NS | Lymphedema (6.5; bilateral) | MDS/AML (16/29) with trisomy 21 | Recurrent pneumonia and ear infections, H influenza meningitis | Current study, Wildin (submitted) |
| Patient 7 | 1 male | De novo | p.Leu332Thrfs*53 | chr3:g.128 202 727-128 202 728ins GGTC GATA2 c.992_993insGACC (p.Leu332Thrfs*53) | LOF ZF(−) | L, M/A | Lymphedema (birth; bilateral) | Pancytopenia (14) with monosomy 7, MDS (18) with monosomy 7 and trisomy 8, AML (18) | No | Current study |
| Patient 8, kindred 13.I.2 | 1 female (mother) | Familial | p.Met1del290 | chr3:g.128 204 043-128 211 896 GATA2 c.1-200_871 + 527del 2033 bp (p.Met1del290) | Null | L, ID | Lymphedema (unilateral left) | Monocytopenia and B-cell, NK-cell, and T-cell lymphocytopenia | Warts (adulthood), no history of infections disseminated MAC, fungal infections, warts, recurrent pneumonias | Current study, Vinh,27 Hsu7 |
| Patient 9, kindred 13.II.1 | 1 male (son) | Familial | p.Met1del290 | chr3:g.128 204 043-128 211 896 GATA2 c.1-200_871 + 527del 2033 bp (p.Met1del290) | Null | M/A | None | MDS (33) with monosomy 7 on all metaphases, trisomy 8, pancytopenia (33) | Current study, Vinh,27 Hsu7 | |
| Patient 10 | 1 male | De novo | p.? | chr3:g.128 202 701A>C GATA2 c.1017 + 2T>G (p.?) | LOF ZF(−) | M/A, ID | None | MDS (19), monocytopenia (19) | Warts, severe acne | Current study |
Patients 1 to 3 belong to the same family, whereas patients 4 to 6 are unrelated and harbor large heterozygous deletions encompassing the entire GATA2 gene and surrounding genes. Patient 5 has been previously described.21 All mutations numbered from ATG start codon of GATA2 NM_032638.4 and NP_116027.2 (February 2009 GRCh37/hg19).
DB(−) indicates DNA binding disrupted; ZF(−), zinc finger 2 fully or partially deleted; LOF, predicted loss of function; L, lymphedema; M/A, MDS and/or AML; ID, immunodeficiency (DCML or MonoMAC); DF, dysmorphic features; and NS, neurologic symptoms (eg, mental retardation, developmental delay).
Experimentally validated.
Common phenotypic features of the 3 persons with large, contiguous de novo deletions encompassing the GATA2 gene and surrounding genes (patients 4-6, Table 1) include mental retardation, developmental delay, and dysmorphic features. Although it cannot be excluded that the deletions in patients 4 and 5 were acquired, this is doubtful because all 3 patients with large deletions have developmental and neurologic disturbances, most likely because of the mutation being present from conception. No other large genomic rearrangements were detected in these patients. Interestingly, the combination of developmental/neurologic features with lymphedema and pancytopenia/MDS/AML in patient 6 is similar to Emberger syndrome (OMIM 614038), characterized by primary lymphedema and MDS, with or without other nonspecific developmental phenotypes.28 All 3 patients with large deletions have pancytopenia/MDS/AML. The minimal deletional overlap between these patients contains only a small number of genes, including GATA2 (supplemental Figure 2), indicating that GATA2 deletion is probably responsible for the hematologic phenotype. Similar to MonoMAC syndrome,7,27 these 3 patients were also susceptible to contracting a characteristic range of persistent infections. The affected mother in a MonoMAC pedigree (kindred 13.I.2, Table 1) with a deletion removing the GATA2 promoter and the first 290 amino acids, probably rendering it a complete LOF allele, also displayed unilateral lymphedema, whereas her son did not at 33 years of age.7 Lymphedema was also a feature of a patient (patient 7, Table 1) carrying a de novo GATA2 frameshift mutation located in the first zinc finger of GATA2 (p.Leu332Thrfs*53) and therefore probable to result in GATA2 LOF. Patient 7 has MDS/AML, but no developmental/neurologic symptoms. Based on these observations and in light of recent reports of MDS/AML with lymphedema,28,29 we hypothesized that lymphedema may be a partially penetrant phenotype associated with germline GATA2 mutations and investigated the role of GATA2 in the lymphatic vasculature.
Gata2 levels and localization in the lymphatic vasculature
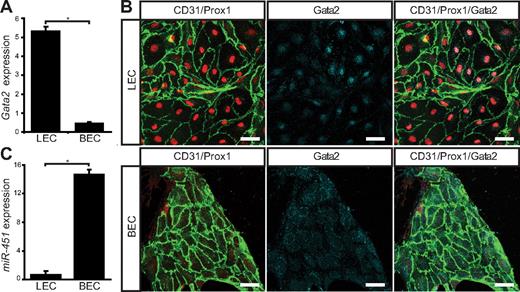
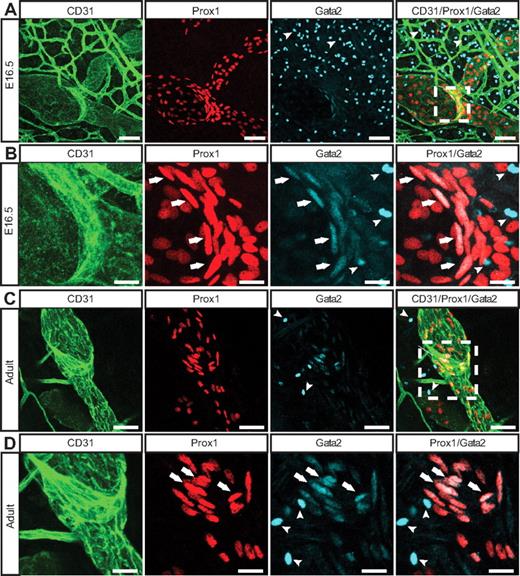
To determine whether loss of GATA2 activity could underlie primary lymphedema, we sought to investigate the role of GATA2 in lymphatic vascular development. We first examined the expression levels and localization of Gata2 mRNA and protein in lymphatic and blood vascular compartments. Our data revealed that Gata2 mRNA was elevated approximately 10-fold in primary lymphatic (LECs), compared with blood vascular (BECs) endothelial cells isolated from the skin of embryonic (Figure 1A) mice and, moreover, that nuclear Gata2 protein was present selectively in isolated LECs, but not BECs (Figure 1B). Nuclear Gata2 protein was also apparent in lymphatic vessels, but not blood vessels, of embryonic and adult tissues (Figure 2). Strikingly, within the lymphatic vasculature, Gata2 was present at particularly high levels in lymphatic vascular valves, identified on the basis of morphology, together with high level Prox1 and CD31 (encoded by Pecam1) immunostaining (Figure 2 arrows). Gata2 protein was obvious in the EC composing lymphatic valves from the onset of valve morphogenesis at approximately E16.5 (Figure 2A-B) and was maintained through adulthood (Figure 2C-D), suggesting that Gata2 might have a key role in programming valve identity, development, and function. High levels of Gata2 protein were also obvious in CD45-positive hematopoietic cells in skin (Figure 2A-D arrowheads; supplemental Figure 3), in accordance with demonstrated roles of Gata2 during hematopoietic development.1,2
Gata2 mRNA and protein levels are elevated in primary embryonic mouse LECs. (A) Quantification of Gata2 mRNA expression levels assessed by real-time RT-PCR in primary dermal LECs and BECs. Error bars represent mean ± SD of 3 replicates, and data are representative of 3 independent cell isolations from 20 to 30 embryos. (B) Primary dermal LECs and BECs cultured for 3 days on fibronectin and immunostained with CD31 (green), Prox1 (red), and Gata2 (cyan). Scale bar represents 30 μm. (C) Quantification of miR-451 expression levels assessed by real-time RT-PCR in primary dermal LECs and BECs. Error bars represent mean ± SD of 3 replicates, and data are representative of 3 independent cell isolations from 20 to 30 embryos. *P < .05 (Student paired t test).
Gata2 mRNA and protein levels are elevated in primary embryonic mouse LECs. (A) Quantification of Gata2 mRNA expression levels assessed by real-time RT-PCR in primary dermal LECs and BECs. Error bars represent mean ± SD of 3 replicates, and data are representative of 3 independent cell isolations from 20 to 30 embryos. (B) Primary dermal LECs and BECs cultured for 3 days on fibronectin and immunostained with CD31 (green), Prox1 (red), and Gata2 (cyan). Scale bar represents 30 μm. (C) Quantification of miR-451 expression levels assessed by real-time RT-PCR in primary dermal LECs and BECs. Error bars represent mean ± SD of 3 replicates, and data are representative of 3 independent cell isolations from 20 to 30 embryos. *P < .05 (Student paired t test).
Localization of Gata2 in the lymphatic vasculature. E16.5 (A-B) and adult (C-D) skin immunostained with anti-CD31 (green), Prox1 (red), and Gata2 (cyan) antibodies. Arrows indicate Gata2 localization in lymphatic vascular valves; and arrowheads, Gata2-positive hematopoietic cells. Boxed regions in panels A and C are shown at higher magnification in panels B and D, respectively. Scale bars represent 30 μm (A,C), 10 μm (B), and 15 μm (D).
Localization of Gata2 in the lymphatic vasculature. E16.5 (A-B) and adult (C-D) skin immunostained with anti-CD31 (green), Prox1 (red), and Gata2 (cyan) antibodies. Arrows indicate Gata2 localization in lymphatic vascular valves; and arrowheads, Gata2-positive hematopoietic cells. Boxed regions in panels A and C are shown at higher magnification in panels B and D, respectively. Scale bars represent 30 μm (A,C), 10 μm (B), and 15 μm (D).
The selective localization of Gata2 protein in lymphatic, but not blood, vessels was unexpected, given that a Gata2GFP reporter mouse in which GFP was knocked in to the Gata2 locus has been reported to display broad GFP expression in cardiac, blood vascular, and lymphatic vascular endothelial cells of the mouse embryo.14 These data suggested that Gata2 mRNA and/or protein levels are differentially controlled in lymphatic and blood vascular endothelial cells and prompted us to investigate potential mechanisms responsible for posttranscriptional Gata2 regulation. To this end, we investigated the levels of miR-451, a microRNA previously shown to target Gata2 in zebrafish,30 in lymphatic and blood vascular endothelial cells. Strikingly, miR-451 levels were elevated by approximately 21-fold in blood vascular, compared with lymphatic vascular, endothelial cells (Figure 1C), providing a potential mechanism for the targeted degradation of Gata2 mRNA and/or prevention of translation of Gata2 protein, in blood vascular endothelial cells.
Gata2 regulates the expression of genes required for valve formation
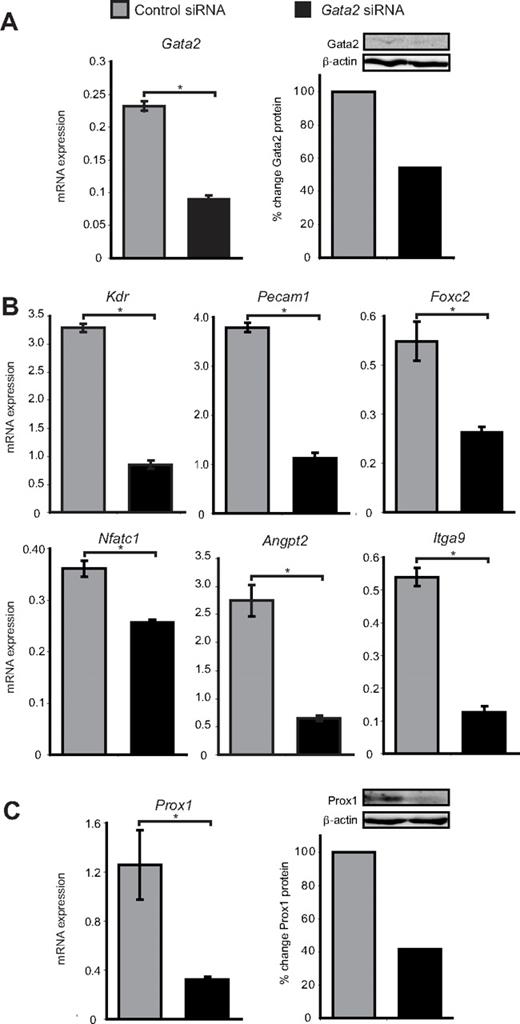
To investigate the role of Gata2 in lymphatic vascular valves, we treated primary LECs isolated from the dermis of E16.5 embryonic mice with Gata2 or control siRNA and assessed the expression levels of genes that have been established to play important roles in valve development. Gata2 mRNA and protein levels were reduced by 60% and 45%, respectively, 48 hours after transfection with Gata2 siRNA (Figure 3A). A corresponding marked decrease in levels of established GATA2 target genes Pecam1, Kdr, and Angpt2 was observed in Gata2, but not control siRNA-treated LECs (Figure 3B). Interestingly, mice deficient in Angpt2 display defects in lymphatic vascular maturation and remodeling together with a deficiency of lymphatic vascular valves,31,32 and CD31 levels are elevated in the LECs that compose lymphatic valves (Figure 2). Levels of FoxC2, Nfatc1, and Itga9, all of which play key roles in lymphatic valve morphogenesis,33-35 were also significantly reduced in Gata2 siRNA-treated LECs (Figure 3B), illustrating that the programming of valve development is probably dramatically affected as a result of GATA2 LOF. Interestingly, Nfatc1 mRNA levels, while reduced, were not changed as substantially after Gata2 knockdown as other genes were (Figure 3B), indicating that expression of this transcription factor in LECs may be regulated by Gata2 indirectly. Taken together, our data illustrate that several of the key genes expressed highly in, and important for, the construction of valves appear to be regulated by Gata2. Intriguingly, Prox1 mRNA and protein levels were reduced by approximately 75% and 60%, respectively, in Gata2 siRNA-treated LECs (Figure 3C) and the level of nuclear Prox1 protein observed in primary LECs correlated with that of Gata2 (Figure 1B). These data suggest that Gata2 activity might be responsible for the elevation of Prox1 protein levels in valves and position Gata2 upstream of Prox1 in the transcriptional hierarchy underlying valve morphogenesis. Overall, our data suggest that Gata2 may control valve development by regulating expression of genes, including Foxc2, Nfatc1, Itga9, Angpt2, Pecam1, and Prox1.
Gata2 target genes are down-regulated after siRNA knockdown of Gata2. (A) Gata2 mRNA and protein levels are substantially reduced in primary embryonic LECs after transfection with Gata2 siRNA, compared with transfection with control siRNA. (B) Quantification of Kdr, Pecam1, Foxc2, Nfatc1, Angpt, and Itga9 mRNA expression levels by real-time RT-PCR in primary LECs transfected with control and Gata2 siRNA. (C) Prox1 mRNA and protein levels are substantially reduced in primary embryonic LECs after transfection with Gata2 siRNA, compared with transfection with control siRNA that has no established targets. Error bars represent mean ± SEM (n = 3), and data are representative of 3 independent cell isolations from 20 to 30 embryos. *P < .05 (Student paired t test). Quantification of protein levels was calculated after normalization with respect to β-actin and expressed as percentage change from controls. Each lane contains equal amounts of protein pooled from 3 separate transfections.
Gata2 target genes are down-regulated after siRNA knockdown of Gata2. (A) Gata2 mRNA and protein levels are substantially reduced in primary embryonic LECs after transfection with Gata2 siRNA, compared with transfection with control siRNA. (B) Quantification of Kdr, Pecam1, Foxc2, Nfatc1, Angpt, and Itga9 mRNA expression levels by real-time RT-PCR in primary LECs transfected with control and Gata2 siRNA. (C) Prox1 mRNA and protein levels are substantially reduced in primary embryonic LECs after transfection with Gata2 siRNA, compared with transfection with control siRNA that has no established targets. Error bars represent mean ± SEM (n = 3), and data are representative of 3 independent cell isolations from 20 to 30 embryos. *P < .05 (Student paired t test). Quantification of protein levels was calculated after normalization with respect to β-actin and expressed as percentage change from controls. Each lane contains equal amounts of protein pooled from 3 separate transfections.
Discussion
Our work has identified heterozygous GATA2 deletions and a GATA2 frameshift mutation in patients with predisposition to MDS/AML, immunodeficiency, and primary lymphedema and uncovered a novel role for GATA2 in lymphatic vascular development and/or function, particularly in regulating the expression of genes important for valve morphogenesis. In contrast to most of the missense and small in-frame GATA2 mutations that we recently described in MDS/AML and MonoMAC/DCML syndrome, the mutations we describe in 3 patients with lymphedema and MDS/AML are either complete or partial gene deletions, or a frameshift mutation, all of which are predicted to result in one complete LOF GATA2 allele and, hence, haploinsufficiency. This finding initially suggested that one explanation for the incomplete penetrance of the lymphedema phenotype could be that missense mutations of GATA2 might retain partial activity, either in terms of DNA binding or interaction with partner proteins, which renders them less disruptive to lymphatic vascular function than heterozygous GATA2 deletion. This is supported by the new family presented here with the p.Thr354Met mutation, bringing to 8 the number of families and de novo occurrences of this mutation and 37 the number of persons with this mutation, none of whom has lymphedema.6-8 An additional patient described here with a novel splice site mutation in GATA2 has MDS, but not lymphedema. With no RNA available to study, it is difficult to predict the effect of this mutation on GATA2 protein. The absence of lymphedema may indicate that this particular mutation is not a total LOF. However, lymphedema is still only a partially penetrant phenotype in the patients with LOF alleles. Moreover, 8 patients have been described with p.Arg398Trp and 2 with different mutations at p.Arg396, none of whom exhibits lymphedema.6-8 This emerging genotype to phenotype correlation may reflect the different GATA2 transcriptional complexes present in lymphatic endothelial versus hematopoietic cells.
While our manuscript was under review, however, familial and de novo mutations in GATA2 were also identified in 2 pedigrees and 6 sporadic cases of Emberger syndrome,36 a disorder characterized by primary lymphedema with MDS/AML.28 Two de novo missense mutations described in Emberger syndrome (p.Arg361Leu and p.Cys373Arg)36 are predicted to grossly affect structure of the second zinc finger and the ability of GATA2 to bind DNA; the p.Cys373Arg mutation disrupts one of the 4 crucial zinc ion coordinating cysteines, replacing it with a zinc-repelling, positively charged arginine, whereas the R361L change is predicted by modeling to disrupt DNA binding (supplemental Figure 4). Both mutants were demonstrated to reduce GATA2-mediated transactivation.36 To date, these are the only patients with GATA2 germline missense mutations who exhibit lymphedema.
Of the remaining 6 mutations reported by Ostergaard et al, 5 were heterozygous frameshift mutations and 1 a nonsense mutation (supplemental Table 2),36 all of which are predicted to result in premature truncation before the DNA binding second zinc finger of GATA2.36 These mutations add to the 4 frameshift mutations found in the MonoMAC/DCML syndrome.7,8 Although Hyde and Liu suggested that there may be alternative translational start sites used in the transcripts derived from these mutant alleles, thereby generating short GATA2 isoforms with differing activities37 (as has been shown in acquired GATA1 mutations in Down syndrome leukemogenesis38 ), the simplest explanation of these alleles is that they are complete LOF alleles and thus haploinsufficient. Our description of a patient harboring a de novo frameshift mutation in the first zinc finger of GATA2 (p.Leu332Thrfs*53) adds to those described in Emberger syndrome patients and provides further evidence that loss of GATA2 function is associated with lymphedema onset.
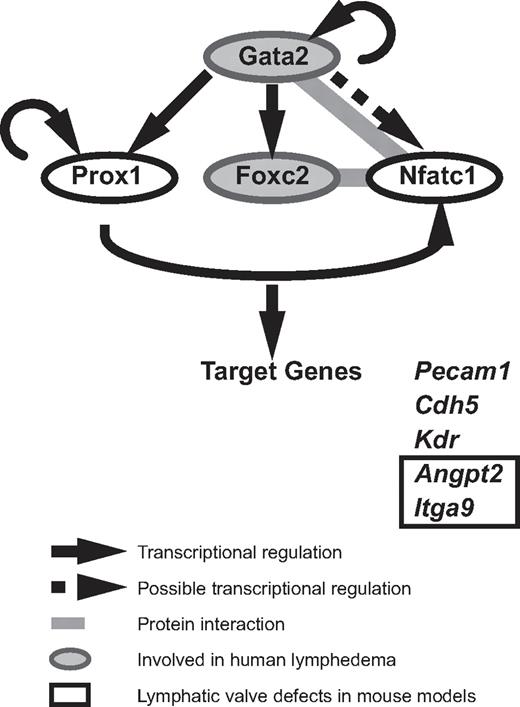
Although GATA2 has long been implicated in vascular development, our data are the first to demonstrate that Gata2 protein is localized selectively in lymphatic vessels and at particularly high levels in the endothelial cells that compose lymphatic vascular valves. The construction of valves in collecting lymphatic vessels is a key event during maturation of the lymphatic vasculature into a hierarchical vascular network34,35 and failure of these specialized structures to form or function to full capacity affects the unidirectional propulsion of lymph from the extremities back to the bloodstream, resulting in lymphedema. High levels of Prox1 and Foxc2 within discrete clusters of cells in the lymphatic capillary plexus between E15 and E16 mark the initiation of lymphatic valve development in the mouse embryo, and both Prox1 and Foxc2 play key roles in valve formation.33,34,39 Recent work has revealed that the transcription factor Nfatc1 coordinately regulates gene expression with Foxc2 and that Nfatc1 function is also required for the construction of lymphatic valves.34 How does GATA2 fit into the transcriptional program responsible for valve development? Our work reveals that Gata2 lies upstream of Prox1 in the transcriptional hierarchy of valve morphogenesis (Figure 4). Nfatc1 has been shown to heterodimerize with Gata2 in myocytes,40 leading to the intriguing possibility that Gata2 might interact with Nfatc1 in LECs, either to join with FoxC2 and Nfatc1 as part of a higher-order transcriptional complex, or alternatively, to displace Nfatc1 from Foxc2/Nfatc1 complexes (Figure 4). Future studies will investigate these possibilities. In hematopoietic stem cells, GATA2 has been reported to coordinately regulate transcription in a transcription factor “triad” composed of GATA2, FLI1, and SCL/TAL1, in which each transcription factor regulates its own transcription, together with transcription of the other 2 triad members.41 Whether FLI1 and SCL/TAL1 are also involved in GATA2 transcriptional complexes in the endothelial cells that compose vascular valves remains to be explored. Despite recent progress that has identified molecules, including ephrinB2,42 integrin-α9, and its ligand fibronectin EIIIA35 and connexins 37, 43, and 47,43 as being important for the construction of lymphatic vascular valves, the upstream signals that initiate the programming of valve development remain poorly defined. Our data implicate GATA2 as an apical regulator of the transcriptional programming of valve development.
A model of Gata2 regulated transcription in LECs. Our data demonstrate that GATA2 regulates the expression of key transcription factors important for valve development. GATA2 lies upstream of Prox1, Foxc2, and Nfatc1. FoxC2 and NFATc1 have been demonstrated to interact together to coordinately regulate transcription in LECs. NFATc1 and Gata2 have been found to heterodimerize in myocytes, suggesting that these transcription factors might interact together in LECs. Gata2, Prox1, FoxC2, and NFATc1 have been demonstrated to bind the promoter and/or regulate the transcription of genes, including Pecam1, Kdr, Cdh5, Angpt2, and Itga9.
A model of Gata2 regulated transcription in LECs. Our data demonstrate that GATA2 regulates the expression of key transcription factors important for valve development. GATA2 lies upstream of Prox1, Foxc2, and Nfatc1. FoxC2 and NFATc1 have been demonstrated to interact together to coordinately regulate transcription in LECs. NFATc1 and Gata2 have been found to heterodimerize in myocytes, suggesting that these transcription factors might interact together in LECs. Gata2, Prox1, FoxC2, and NFATc1 have been demonstrated to bind the promoter and/or regulate the transcription of genes, including Pecam1, Kdr, Cdh5, Angpt2, and Itga9.
The selective localization of Gata2 in lymphatic vessels and within the nuclei of primary mouse lymphatic, but not blood vascular endothelial cells, reveals previously unrecognized complexity in the posttranscriptional and/or posttranslational mechanisms that control Gata2 protein levels in endothelial cells. Our finding that miR-451 levels are dramatically elevated in blood vascular endothelial cells compared with LECs provides one potential mechanism contributing to post-transcriptional Gata2 regulation and resulting in diminished levels of Gata2 protein in blood vessels. Given that recent work has revealed that the programs of valve development in lymphatic vessels and veins are remarkably conserved,44 it will be interesting to determine whether GATA2 is also localized to venous valves. Our results revealing that Gata2 protein is present selectively in lymphatic vessels may go some way to explaining why vascular phenotypes have not been observed in Gata2−/− mice before their embryonic death at approximately E10. Interestingly, embryonic edema was a prominent feature of mice in which Gata2 was conditionally excised using a FoxG1-Cre line, chosen to delete selectively in the otic epithelium and mesenchyme but found to be broadly active in most embryonic tissues.45 Conditional deletion of Gata2 specifically in endothelial cells will go further toward delineating the role of this transcription factor in blood and lymphatic vascular development.
What are the signals responsible for switching GATA2 “on” in lymphatic vascular valves? We and others34 have observed that lymphatic vascular valves often form near branchpoints of the lymphatic vasculature and recent, elegant work has revealed that the endothelial cells immediately upstream and downstream of venous and lymphatic valves exhibit morphologic differences; endothelial cells upstream of valves are organized in an elongated shape, whereas endothelial cells downstream of valves exhibit rounded morphology.44 These observations suggest that differences in directional fluid flow and shear stress could be responsible for initiating valve development. In support of this hypothesis, GATA2 localization in endothelial cells has been previously shown to be responsive to mechanical stimuli.46 Molecules with a documented role in mechanotransduction in endothelial cells include ion channels, G-proteins, and cytoskeletal and cell junction elements,47 although which of these pathways acts to regulate GATA2 levels in lymphatic vascular valves remains to be investigated. Additional signals that warrant further investigation with respect to regulating GATA2 levels in lymphatic vascular valves include the BMP448 and NOTCH49 signaling pathways, both of which have been established to activate the Gata2 promoter and are important during vascular development.
The data presented in this paper provide compelling evidence that GATA2 mutations are associated with human primary lymphedema and that GATA2 is a critical player in the development and/or maintenance of lymphatic vascular valves. The mutations found in our patients, interpreted together with the 6 GATA2 Emberger syndrome frameshift and nonsense mutations,36 strongly suggest that GATA2 haploinsufficiency, as opposed to missense mutations or small in-frame deletions, preferentially predispose to the lymphedema phenotype. Lymphedema in these patients may present at different ages of onset (birth to 44 years after operation), be of differing severities, even among family members carrying the same GATA2 mutation, be unilateral or bilateral, and also be partially penetrant (patients 8 and 9). Intriguingly, of the 5 unilateral lymphedema patients, 4 showed left-sidedness (supplemental Table 2).
The different phenotypes seen in patients with heterozygous germline GATA2 mutations can be partially explained by the way the patient data were ascertained. Hahn et al assessed patients on the basis of MDS/AML,6 Hsu et al on the basis of immunodeficiency and infectious diseases,7 and Ostergaard et al on the basis of primary lymphedema and other Emberger syndrome phenotypes.36 The phenotypes that present in individual patients may also be a consequence of allele-specific effects and/or modifier genes and/or or environmental interactions. The hematopoietic stem cell defects in all GATA2 patients clearly lead to cytopenias, and these cytopenias may lead to, and be exacerbated by, infections as an environmental stress. Susceptibility to various infections may also be exacerbated by defects in the development and/or maintenance of lymphatic vascular valves, resulting in altered lymph transport, particularly in the skin; chronic infections of skin appear to be more prevalent in patients with lymphedema (supplemental Table 2). The different ages of onset and partial penetrance of the lymphedema phenotype may also reflect the amount of stress under which a patient's lymph valves are placed, which may be partially mechanical and also related to infections. Substantial additional work is still required to fully understand the phenotypic consequences of various GATA2 mutations. Further defining the upstream signals that activate GATA2, together with the transcriptional program regulated by GATA2 that is important for valve development, will no doubt expand our understanding of valve morphogenesis and may provide new targets to which novel therapeutics aimed at improving lymphatic vascular function could be designed.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and families for their participation in our studies, K. Wicks and staff of the SA Pathology Veterinary Services division for animal husbandry, and A. Bert for miR-451 quantification.
This work was supported by the National Health and Medical Research Council of Australia (project grant 1002317, H.S.S.; project grant 626959, N.L.H.; and fellowship 461204, H.S.S.), the Cancer Council of South Australia (H.S.S. and N.L.H.), MedVet Pty Ltd (H.S.S. and N.L.H.), and National Institutes of Health (grants R01DK058161 and T32GM007266, M.S.H.).
National Institutes of Health
Authorship
Contribution: J.K., G.A.S., Y.J.L., A.S., T.W., M.S.H., C.N.H., J.C.-R., A.P.H., H.S.S., and N.L.H. designed experiments; J.K., G.A.S., J.A.R., C.-E.C., M.B., M.Y.Z., T.W., and C.N.H. performed experiments and analyzed data; R.S.W., S.D., C.V.F., P.G.B., A.S., S.M.H., and D.D.H. provided clinical care and patient samples and revised the manuscript; and J.K., G.A.S., Y.J.L., C.N.H., H.S.S., and N.L.H. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: J.A.R. is an employee of Signature Genomic Laboratories, a subsidiary of PerkinElmer Inc. The remaining authors declare no competing financial interests.
Correspondence: Natasha L. Harvey, Division of Haematology, Centre for Cancer Biology, SA Pathology, PO Box 14, Rundle Mall, Adelaide, 5000, South Australia, Australia; e-mail: natasha.harvey@health.sa.gov.au; and Hamish S. Scott, Department of Molecular Pathology, Centre for Cancer Biology, SA Pathology, PO Box 14, Rundle Mall, Adelaide, 5000, South Australia, Australia; e-mail: hamish.scott@health.sa.gov.au.
References
Author notes
J.K., G.A.S., Y.J.L., and C.N.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal