In this issue of Blood, de Bruin and colleagues demonstrate the ability of IFN-γ to influence the binary cell fate choices of granulocytic-monocytic progenitors (GMPs) during viral infection, favoring monocytic over the granulocytic differentiation.1 This work provides mechanistic insights and a better understanding on how hematopoiesis can be remodeled during infections.
Infections are the most common stressors of the hematopoietic system. The ability of the BM to respond to infections by expanding the progenitor pool to produce more differentiated effector cells is a critical feature of the host's defense, which translates into the difference between resolving an infection or succumbing to it.
For many years, studies have been focused on understanding the functions of critical effector cells of the innate and adaptive immunity, such as neutrophils, monocytes, macrophages, and lymphocytes. Recently, new conceptual and technical advances, including the availability of a wide range of genetic models, have led investigators to look at the immune response from a new angle, opening a window on the interface between stem cell biology and immunity. Recent studies have shown that hematopoietic stem cells (HSCs) and multipotential progenitors play a critical role in host defense and their behavior can determine the abundance of specific lineages by shaping, at the very origin, the hematopoietic response to infection.2-4
This elegant work by de Bruin et al shows that IFN-γ can interfere with the binary cell fate decision of GMPs to differentiate into monocytes or granulocytes, compelling monocytic differentiation. Importantly, the authors show that loss of granulocytic differentiation does not occur by default, but by IFN-γ–mediated active suppression of G-CSF–triggered intracellular responses.
This finding stems from the initial observation that transgenic mice overexpressing CD70 (CD70TG) have an increased production of monocytes over granulocytes. In these mice, overexpression of CD70 in B cells causes a strong activation and expansion of Th1 effector T cells, via CD70-CD27 interaction, and results in the secretion of high levels of IFN-γ, pointing to a role for IFN-γ as inducer of monocytic differentiation in vivo. This hypothesis was confirmed using several complementary in vivo models. The authors show that normal monocyte levels can be restored in CD70TG in the absence of IFN-γ, and that monocytosis can be induced either by T-cell adoptive transfer in CD70TG/CD27−/− mice or by injection of a CD40 agonist in WT mice. As Th1 activation is an adaptive immune response occurring during viral infections, the authors tested the physiologic relevance of their findings in a model of Lymphocytic Choriomeningitis Virus (LCMV) infection. Using this model in conjunction with IFN-γ−/− mice, they confirmed that IFN-γ is required for induction of monopoiesis during infection. Strikingly, these experiments also revealed a requirement of IFN-γ for granulopoiesis suppression.
IFN-γ is a well-known critical mediator of immunity and inflammation5 and its activity has been investigated for more than 3 decades. IFN-γ plays a central role in macrophage and T-cell activation during inflammation and infection, and a few studies have reported that IFN-γ to enhance monocytic differentiation in vitro.6,7 Then why is the present work novel and relevant? Because it addresses for the first time the role of IFN-γ in monocytic differentiation in vivo. It also unveils a previously unrecognized role for IFN-γ in suppressing granulopoiesis during infection, providing novel information on the molecular mechanisms involved in this process and in the regulation of monocytic versus granulocytic differentiation.
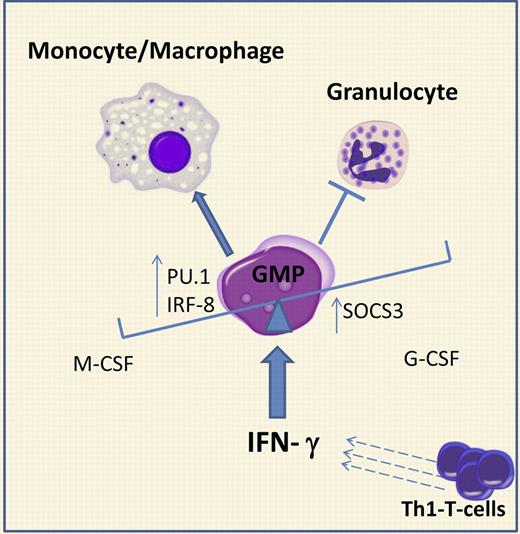
Under homeostatic conditions, the balance between monocytes and granulocytes is regulated by M-CSF and G-CSF, respectively. Each of these cytokines has been shown to be required and sufficient to instruct lineage choice in GMPs in vitro.8 Here, de Bruin et al demonstrate that IFN-γ interferes with this balance and forces myelopoiesis toward monocytic lineage using 2 mechanisms, as shown in the figure: (1) increasing the levels of PU.1 and IRF-8, transcription factors required for GMP cells to commit to the monocytic-macrophage lineage; and (2) inducing expression of SOCS3, which strongly antagonizes G-CSF signaling, required for steady-state and emergency granulopoiesis. Thus, this work provides a good example of how an inflammatory cytokine can override the physiologic regulation of binary cell fate decisions and re-direct hematopoietic differentiation, enhancing one cell fate while suppressing another.
IFN-γ redirects cell fate decisions in granulocytic-monocytic progenitors (GMPs) during infection. In homeostatic conditions, G-CSF and M-CSF regulate the monocytic and granulocytic output from GMPs. During viral infections, IFN-γ, produced by activated Th1 T cells, affects GMP differentiation by: (1) up-regulation of the expression of PU.1 and IRF-8, transcription factors driving monocytic differentiation, and (2) induction of SOCS3, which antagonizes G-CSF–dependent STAT3 phosphorylation. The overall result is a skewing of myelopoiesis toward monopiesis at the expense of granulopiesis.
IFN-γ redirects cell fate decisions in granulocytic-monocytic progenitors (GMPs) during infection. In homeostatic conditions, G-CSF and M-CSF regulate the monocytic and granulocytic output from GMPs. During viral infections, IFN-γ, produced by activated Th1 T cells, affects GMP differentiation by: (1) up-regulation of the expression of PU.1 and IRF-8, transcription factors driving monocytic differentiation, and (2) induction of SOCS3, which antagonizes G-CSF–dependent STAT3 phosphorylation. The overall result is a skewing of myelopoiesis toward monopiesis at the expense of granulopiesis.
The physiologic and clinical relevance of these findings is evident. During viral and intracellular pathogen infections, the ability of the hematopoietic system to coordinate a Th1 T-cell response, while simultaneously shifting myelopoiesis toward monopoiesis via IFN-γ, is critical for the resolution of the infection, as monocytes and activated macrophages are central for pathogen clearance and myco-bacteria killing. However, suppression of granulopoiesis by IFN-γ can have negative implications. It is known that IFN-γ does not always favor the host immune response against bacterial pathogens and, indeed, its production is often suppressed by negative feedback loops (such as IL-10). In light of these novel findings, it is likely that the detrimental effect of IFN-γ during bacterial infection is because of its inhibitory effects on granulopoiesis. In fact, a key component of the host innate response to bacterial pathogens is represented by the neutrophil granulocytes defense system. Bacterial infection induces rapid mobilization of neutrophils to the site of infection and this reactive neutrophilia is maintained by accelerated production and differentiation of granulocytes in the bone marrow. Thus, in contrast to viral infection, the shift of myelopoiesis toward granulopoiesis is critical for resolution of bacterial infection.
Neutropenia is a poor prognostic factor in bacterial infections and often characteristic of severe sepsis with fatal outcome. Despite its clinical relevance, the causes of neutropenia in sepsis have been little investigated and, for a long time, depletion of neutrophils has been commonly accepted as the mere consequence of consumption during the antibacterial response. Pioneer work by Santangelo et al documented signs of myelosuppression and a shift toward monopoiesis at expense of granulopoiesis in murine models of severe sepsis.9 Recently, it is becoming increasingly evident that causes of neutropenia in sepsis include not only bacterial consumption and apoptosis,10 but also decreased neutrophil production by the bone marrow, such as defective generation of myeloid progenitors at the HSC level, as shown by our group.3
The ability of IFN-γ to suppress G-CSF response by inducing the signal transduction antagonist SOCS3 can also explain why septic patients are neutropenic despite normal levels of G-CSF or are insensitive to G-CSF treatment. Similarly, this mechanism could also account for increased susceptibility of bacterial superinfection in patients after viral infections. On the bases of the findings of de Bruin et al, it will be clinically relevant to investigate whether patients with bacterial infection exhibiting neutropenia have high levels of IFN-γ and whether detection of high IFN-γ levels could be a negative prognostic factor in severe bacterial infection progressing to sepsis. Although the clinical impact of this work remains to be seen, these observations have moved our understanding of how the hematopoietic system redirects lineage differentiation during infection a step forward and also open the possibility of targeting the IFN-γ pathway to restore neutrophil differentiation during bacterial infections.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal