Abstract
VWF and ADAMTS13 are major determinants of platelet adhesion after vessel injury. In the present study, we aimed to determine whether VWF or ADAMTS13 plasma antigen levels influence the risks of ischemic stroke (IS) or myocardial infarction (MI) in young women and how these risks are affected by oral contraceptive (OC) use. VWF and ADAMTS13 plasma antigen levels were measured in a frequency-matched case-control study of 1018 young (18-49 years) women including 175 IS patients and 205 MI patients. Increasing levels of VWF and decreasing levels of ADAMTS13 were associated with the risk of IS and MI in a dose-dependent manner. Having both high VWF and low ADAMTS13 resulted in an odds ratio (OR) of 6.9 (95% confidence interval [95% CI], 2.0-23.0) for IS and 11.3 (95% CI, 3.6-35.2) for MI. Use of OCs increased the risk of IS and MI associated with high VWF (OR = 12; 95% CI, 5.5-26.2 and OR = 7.5, 95% CI, 3.6-15.7, respectively) and the risk of IS associated with low ADAMTS13 (OR = 5.8, 95% CI, 2.7-12.4). We conclude that high VWF and low ADAMTS13 plasma levels both increase the risk of IS and MI. The risks associated with high VWF or low ADAMTS13 levels are further increased by the use of OCs.
Introduction
VWF is a plasma glycoprotein synthesized by endothelial cells and megakaryocytes1 ; it is a major determinant of platelet adhesion after vessel injury and consequently of clot formation1 and circulates in plasma at approximately 10 μg/mL as multimers with a molecular weight (MW) ranging from 500-20 000 kDa.2 High-MW forms of VWF are stored in Weibel-Palade bodies in endothelial cells and also in platelet α-granules.3,4 These VWF stores are secreted into plasma after endothelium or platelet activation.5,6 The multimeric composition of plasma VWF is regulated by ADAMTS13 (a disintegrin and metalloproteinase with the thrombospondin type I repeat 13), which preferentially cleaves the large VWF multimers into smaller, less prothrombotic species. ADAMTS13 is a metalloproteinase synthesized mainly by hepatocytes, but also by endothelial cells and megakaryocytes.7-9 It circulates in plasma at a concentration of approximately 1 μg/mL.10 The clinical importance of VWF and ADAMTS13 in regulating hemostasis is highlighted by the bleeding and thrombotic diseases associated with their respective deficiencies. VWD is characterized by a qualitative or quantitative deficiency in VWF resulting in impaired platelet tethering. This is the most common inherited bleeding disorder in humans.11 Conversely, thrombotic thrombocytopenic purpura, which is characterized by a deficiency in ADAMTS13, results in the presence of hyperreactive plasma VWF that leads to thrombotic microangiopathy.12
Cardiovascular disease (CVD) is a leading cause of disability and death in both developed and developing countries, and is predicted to affect an increasing number of people worldwide over the next decades.13 Ischemic stroke (IS) and myocardial infarction (MI) are among the most common CVD manifestations. Prospective studies investigating the risk of CVD associated with increased VWF antigen levels have been performed, generally in individuals over the age of 45 years. Some prospective studies have investigated the risk of coronary heart disease, atrial fibrillation, or IS and did not find an association with VWF antigen levels14-17 ; however, other studies investigating the risk of CVD, angina pectoris, hemorrhagic stroke, or IS did find this association.17-20 Several case-control studies have been carried out, and, although some controversy exists, many report an association of high VWF antigen levels with both IS21-23 and MI.24-27 However, in certain case-control studies, blood sampling was performed when the acute phase of the thrombotic event may have still been prevalent. This makes it difficult to determine whether the observed increase in VWF levels are a cause or a consequence of the event, because it has been shown that VWF plasma levels are increased during the acute phase after stroke or MI, possibly as a consequence of damage to the endothelium.28,29 This may even be the case in prospective studies, in which VWF levels could be the result of vessel wall damage that predisposes to disease.
A small number of studies have investigated the association of ADAMTS13 with IS and MI, with conflicting outcomes. Studies investigating ADAMTS13 antigen levels during the acute phase (0-14 days) of MI found decreased levels of ADAMTS13.26,30 It is, however, difficult to establish whether this was a cause or a consequence of the event. Other studies evaluating the association between ADAMTS13 antigen levels and MI between 1 and 9 months after the event found an association with either low ADAMTS1325,31 or high ADAMTS1324 plasma levels. Only 1 study looked at the association between ADAMTS13 antigen levels and a subgroup of IS patients, and found no association.31
In the present study, we have reinvestigated whether plasma VWF and ADAMTS13 levels are associated with the risk of IS and MI in the previously published RATIO (Risk of Arterial Thrombosis in Relation to Oral Contraceptives) case-control study.32-34 This study included only young women (18-49 years), so the findings were presumably less complicated by age-related deterioration of the vasculature. Blood was taken well after the acute phase of the event, meaning that VWF and ADAMTS13 antigen levels should have returned to those before the event, ensuring they were not influenced by acute-phase postevent endothelial damage. We also investigated the combined effect of both VWF and ADAMTS13 levels on the risks of IS and MI, and how the risk was affected by use of oral contraceptives (OCs).
Methods
Study design and participants
Study design and details of the RATIO case-control study have been extensively described elsewhere.32-34 Briefly, women between 18 and 49 years of age with a first event of IS (n = 175) or MI (n = 205) were included. IS was diagnosed based on clinical symptoms, neurologic examination, and computed tomography or magnetic resonance imaging. Exclusion criteria were hemorrhagic stroke, transient ischemic attack, venous sinus thrombosis, carotid artery dissection, history of cardiovascular or cerebrovascular disease, severe illness, cognitive impairment, and aphasia. MI was diagnosed based on clinical symptoms, elevated cardiac-enzyme levels, and electrocardiographic changes. Blood samples were collected after a median of 95 months (range, 23-146 months) for IS and 69 months (range, 38-117 months) for MI, ensuring that plasma proteins should not have been influenced by any acute-phase protein response to the thrombotic event. The control group (n = 638) was frequency matched based on age, area of residence, and index year. Plasma samples from 167 IS patients, 202 MI patients, and 626 patients were available for analysis in the present study. The presence of conventional risk factors for CVD, such as hypertension, hypercholesterolemia, diabetes mellitus, and smoking, and the use of OCs were based on the year before the event/index year. Women using first-, second-, or third-generation OCs were included in the RATIO study.32-34 Our study protocol was approved by the ethical committees of the participating hospitals and informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
VWF ELISA
VWF plasma antigen levels were determined through an in-house ELISA. A polyclonal rabbit anti-VWF Ab (3.1 μg/mL; Dako) was immobilized in a 96-well Maxisorp microplate (Nunc) in 50mM carbonate buffer, pH 9.6, at 4°C overnight. Washing steps were performed in triplicate with 300 μL/well of PBS with 0.1% Tween-20 (PBST) between each step. All steps were carried out at room temperature on a shaker. Wells were blocked with 2.5% BSA (Sigma-Aldrich) in PBST for 2 hours. A standard curve of 0-125 ng/mL was made with normal control plasma (Technoclone), and samples were diluted 1:320 in 1% BSA in PBST and added to the wells in triplicate. Bound VWF was detected with 1.1 μg/mL of HRP-conjugated polyclonal Ab against VWF (Dako) for 1 hour. The plate was developed with 100 μL/well of o-phenylenediamine dihydrochloride (Sigma-Aldrich) for 3 minutes, and the reaction was stopped with 50 μL/well of 2.5M H2SO4 and the absorbance read at 492 nm. The inter- and intra-assay coefficients of variation were 6.4% and 6.3%, respectively.
ADAMTS13 ELISA
ADAMTS13 plasma antigen levels were determined with an in-house ELISA as described previously.24 A polyclonal rabbit Ab (5 μg/mL, depleted of anti–TSP2-4 Abs) against ADAMTS13 was immobilized in a 96-well Maxisorp microplate (Nunc) in 50mM carbonate buffer, pH 9.6, at 4°C overnight. Washing steps were performed in triplicate with 300 μL/well of PBST between each step. All incubations were carried out at room temperature on a shaker. Wells were blocked with 2.5% BSA in PBS for 2 hours. A standard curve of 0-108 ng/mL was made with normal control plasma (Technoclone) and samples were diluted 1:25 in 1% BSA in PBS and added to the wells in triplicate. Bound ADAMTS13 was detected with 0.2 μg/mL of biotinylated polyclonal Ab against the TSP2-4 domains of ADAMTS13 for 2 hours. Wells were incubated with streptavidin-HRP (GE Healthcare) diluted 1:1000 for 1 hour and the plate was developed with 100 μL/well of o-phenylenediamine dihydrochloride (Sigma-Aldrich) for 10 minutes. The reaction was stopped with 50 μL/well of 2.5M H2SO4 and the absorbance was read at 492 nm. Inter- and intra-assay coefficients of variation were 6.9% and 7.3%, respectively.
Statistical analysis
Odds ratios (ORs) and corresponding 95% confidence intervals (95% CI) were calculated as measures of relative risk for IS and MI by unconditional logistic regression. The lowest quartile and < 90th percentile (p; VWF analyses), or the highest quartile and > p10 (ADAMTS13 analyses) of the control group was used as a reference category. Adjustment for stratification variables (eg, age, year of event/index year, and area of residence) was made when determining the first model, OR1. Additional adjustments for potential confounders (eg, hypercholesterolemia, hypertension, diabetes mellitus, and smoking) were included in the second model, OR2. To assess the risk of IS and MI associated with the presence of multiple risk factors (eg, VWF, ADAMTS13, and OC), dummy variables were created. To calculate the joint risk conferred by these risk factors, < p90 was used as a cutoff for VWF and > p10 as a cutoff for ADAMTS13. The joint category, representing no exposure to any of these risks, was used as a reference category. All statistical analyses were performed using SPSS Version 19.0 software.
Results
Table 1 shows the baseline characteristics of the individuals in the control, IS, and MI groups. As expected, the prevalence of known risk factors such as hypercholesterolemia, hypertension, diabetes mellitus, and smoking was higher in the 2 case groups than in the control group.
Baseline characteristics of participants of the RATIO case-control study
| . | IS (n = 175) . | MI (n = 205) . | Control (n = 638) . |
|---|---|---|---|
| Median age, y | 39 | 43 | 39 |
| Caucasian ethnicity | 167 (95%) | 195 (95%) | 602 (94%) |
| OC use* | 92 (53%) | 81 (40%) | 213 (33%) |
| Hypercholesterolemia* | 14 (8%) | 21 (10%) | 19 (3%) |
| Hypertension* | 50 (29%) | 53 (26%) | 40 (6%) |
| Diabetes mellitus* | 7 (4%) | 10 (5%) | 10 (2%) |
| Smoking* | 101 (58%) | 169 (82%) | 270 (42%) |
| . | IS (n = 175) . | MI (n = 205) . | Control (n = 638) . |
|---|---|---|---|
| Median age, y | 39 | 43 | 39 |
| Caucasian ethnicity | 167 (95%) | 195 (95%) | 602 (94%) |
| OC use* | 92 (53%) | 81 (40%) | 213 (33%) |
| Hypercholesterolemia* | 14 (8%) | 21 (10%) | 19 (3%) |
| Hypertension* | 50 (29%) | 53 (26%) | 40 (6%) |
| Diabetes mellitus* | 7 (4%) | 10 (5%) | 10 (2%) |
| Smoking* | 101 (58%) | 169 (82%) | 270 (42%) |
In the year before event/index year.
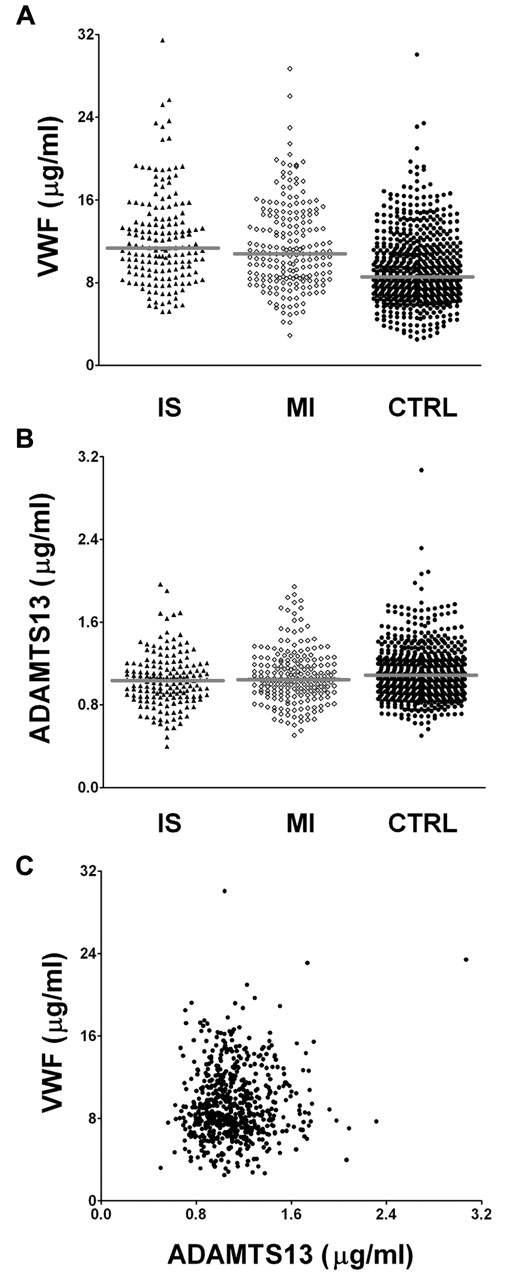
Figure 1 shows the individual VWF (Figure 1A) and ADAMTS13 (Figure 1B) Ag levels in patients with either IS or MI and in the control group. The median levels of VWF plasma concentration in IS patients, MI patients, and controls were 11.4, 10.8, and 8.6 μg/mL, respectively. Median levels of ADAMTS13 were 1.0, 1.0, and 1.1 μg/mL, respectively, in these 3 groups. There was no association between VWF antigen levels and ADAMTS13 antigen levels in the controls (Figure 1C), which is in agreement with previously published data.24,25,31
VWF and ADAMTS13 plasma levels. VWF and ADAMTS13 plasma levels in controls (●), IS patients (▴), and MI patients (♢). (A) VWF plasma levels. (B) ADAMTS13 plasma levels. (C) VWF plasma levels of controls plotted against ADAMTS13 plasma levels. No significant correlation between VWF and ADAMTS13 Ag levels was observed (r = 0.07 by Pearson correlation coefficient). CTRL indicates controls.
VWF and ADAMTS13 plasma levels. VWF and ADAMTS13 plasma levels in controls (●), IS patients (▴), and MI patients (♢). (A) VWF plasma levels. (B) ADAMTS13 plasma levels. (C) VWF plasma levels of controls plotted against ADAMTS13 plasma levels. No significant correlation between VWF and ADAMTS13 Ag levels was observed (r = 0.07 by Pearson correlation coefficient). CTRL indicates controls.
Increased levels of VWF conferred an increased risk for both IS and MI (Table 2). The highest quartile (Q4) of VWF conferred an OR1 of 5.9 (95% CI, 3.0-11.7) for IS and an OR1 of 3.7 (95% CI, 2.1-6.5) for MI. Further adjustment (OR2) for potential confounders did not change the results appreciably.
Risk of IS and MI in relation to VWF and ADAMTS13 plasma levels
| . | Controls, n . | IS . | MI . | ||||
|---|---|---|---|---|---|---|---|
| Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | ||
| VWF Q1 | 156 | 13 | 1 (ref) | 1 (ref) | 20 | 1 (ref) | 1 (ref) |
| VWF Q2 | 157 | 22 | 1.4 (0.7-3.1) | 1.6 (0.7-3.6) | 35 | 1.6 (0.9-3.0) | 1.7 (0.9-3.4) |
| VWF Q3 | 157 | 50 | 3.8 (1.9-7.6) | 4.2 (2.0-9.0) | 60 | 2.5 (1.4-4.5) | 3.1 (1.6-5.9) |
| VWF Q4 | 156 | 82 | 5.9 (3.0-11.7) | 6.7 (3.2-13.8) | 87 | 3.7 (2.1-6.5) | 4.2 (2.2-8.0) |
| ADAMTS13 Q1 | 156 | 61 | 3.1 (1.8-5.5) | 3.1 (1.6-5.8) | 63 | 1.8 (1.1-3.0) | 1.4 (0.8-2.4) |
| ADAMTS13 Q2 | 157 | 45 | 2.4 (1.4-4.4) | 3.0 (1.6-5.7) | 50 | 1.2 (0.8-2.0) | 0.9 (0.5-1.6) |
| ADAMTS13 Q3 | 157 | 33 | 1.5 (0.8-2.7) | 1.7 (0.9-3.3) | 44 | 1.0 (0.6-1.7) | 0.8 (0.5-1.5) |
| ADAMTS13 Q4 | 156 | 28 | 1 (ref) | 1 (ref) | 45 | 1 (ref) | 1 (ref) |
| . | Controls, n . | IS . | MI . | ||||
|---|---|---|---|---|---|---|---|
| Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | ||
| VWF Q1 | 156 | 13 | 1 (ref) | 1 (ref) | 20 | 1 (ref) | 1 (ref) |
| VWF Q2 | 157 | 22 | 1.4 (0.7-3.1) | 1.6 (0.7-3.6) | 35 | 1.6 (0.9-3.0) | 1.7 (0.9-3.4) |
| VWF Q3 | 157 | 50 | 3.8 (1.9-7.6) | 4.2 (2.0-9.0) | 60 | 2.5 (1.4-4.5) | 3.1 (1.6-5.9) |
| VWF Q4 | 156 | 82 | 5.9 (3.0-11.7) | 6.7 (3.2-13.8) | 87 | 3.7 (2.1-6.5) | 4.2 (2.2-8.0) |
| ADAMTS13 Q1 | 156 | 61 | 3.1 (1.8-5.5) | 3.1 (1.6-5.8) | 63 | 1.8 (1.1-3.0) | 1.4 (0.8-2.4) |
| ADAMTS13 Q2 | 157 | 45 | 2.4 (1.4-4.4) | 3.0 (1.6-5.7) | 50 | 1.2 (0.8-2.0) | 0.9 (0.5-1.6) |
| ADAMTS13 Q3 | 157 | 33 | 1.5 (0.8-2.7) | 1.7 (0.9-3.3) | 44 | 1.0 (0.6-1.7) | 0.8 (0.5-1.5) |
| ADAMTS13 Q4 | 156 | 28 | 1 (ref) | 1 (ref) | 45 | 1 (ref) | 1 (ref) |
ORs were calculated by logistic regression and were adjusted for age, year of event/index year, and area of residence. OR2 values were also adjusted for hypercholesterolemia, hypertension, diabetes mellitus, and smoking.
Q indicates quartile; and ref, reference.
Decreased levels of ADAMTS13 conferred an increased risk for both IS and MI (Table 2). An OR1 of 3.1 (95% CI, 1.8-5.5) for IS and an OR1 of 1.8 (95% CI, 1.1-3.0) for MI was conferred by the lowest quartile (Q1) of ADAMTS13. After adjustment, the OR2 for IS did not change, although the OR2 for MI decreased. The risks of IS and MI were also calculated by using p90 for VWF and p10 for ADAMTS13 as cutoff values. The OR1 values for high VWF for IS and MI were 3.2 (95% CI, 1.9-5.3) and 3.0 (95% CI, 1.9-4.7), respectively, and the OR1 values for low ADAMTS13 were 2.3 (95% CI, 1.3-4.0) and 2.0 (1.2-3.2), respectively. Further adjustment (OR2) did not change the estimates appreciably.
Because VWF and ADAMTS13 are functionally linked to each other in vivo, we investigated whether having both high VWF levels and low ADAMTS13 levels further increases the risk of IS and MI. The second row in Table 3 shows the OR1 and OR2 for IS and MI conferred by low ADAMTS13 Ag levels only, and the third row shows the OR1 and OR2 conferred by high VWF levels only. These results were similar to those obtained before stratification, suggesting that VWF and ADAMTS13 each act as risk factors. More importantly, Table 3 shows that the joint effect of high VWF and low ADAMTS13 levels increased the risk of IS and MI, resulting in an OR1 of 6.9 (95% CI, 2.0-23.0) and 11.3 (95% CI, 3.6-35.2), respectively (row 4). These associations were also seen after further adjustment.
Risk of IS and MI following combination of 2 risk factors
| Exposure 1 . | Exposure 2 . | Controls, n . | IS . | MI . | ||||
|---|---|---|---|---|---|---|---|---|
| Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | |||
| VWF < p90 | ADAMTS13 > p10 | 507 | 107 | 1 (ref) | 1 (ref) | 130 | 1 (ref) | 1 (ref) |
| VWF < p90 | ADAMTS13 ≤ p10 | 57 | 20 | 2.2 (1.2-4.1) | 2.0 (1.1-3.8) | 21 | 1.6 (0.9-2.8) | 1.4 (0.8-2.5) |
| VWF ≥ p90 | ADAMTS13 > p10 | 57 | 31 | 3.2 (1.8-5.5) | 3.1 (1.7-5.5) | 39 | 2.6 (1.6-4.2) | 2.6 (1.6-4.3) |
| VWF ≥ p90 | ADAMTS13 ≤ p10 | 5 | 9 | 6.9 (2.0-23.0) | 5.8 (1.7-20.2) | 12 | 11.3 (3.6-35.2) | 9.8 (3.1-30.9) |
| VWF < p90 | No OC use | 372 | 65 | 1 (ref) | 1 (ref) | 92 | 1 (ref) | 1 (ref) |
| VWF < p90 | OC use | 192 | 62 | 2.2 (1.3-3.5) | 2.0 (1.2-3.3) | 59 | 2.2 (1.5-3.4) | 2.1 (1.4-3.3) |
| VWF ≥ p90 | No OC use | 45 | 14 | 1.8 (0.9-3.8) | 1.6 (0.8-3.5) | 29 | 2.7 (1.5-4.7) | 2.8 (1.5-5.0) |
| VWF ≥ p90 | OC use | 17 | 26 | 12.0 (5.5-26.2) | 11.4 (5.2-25.3) | 22 | 7.5 (3.6-15.7) | 7.1 (3.3-15.3) |
| ADAMTS13 > p10 | No OC use | 384 | 69 | 1 (ref) | 1 (ref) | 103 | 1 (ref) | 1 (ref) |
| ADAMTS13 > p10 | OC use | 180 | 69 | 2.6 (1.7-4.3) | 2.6 (1.6-4.1) | 66 | 2.6 (1.7-3.9) | 2.5 (1.6-3.8) |
| ADAMTS13 ≤ p10 | No OC use | 33 | 10 | 2.0 (0.9-4.6) | 1.8 (0.8-4.3) | 18 | 2.5 (1.3-4.9) | 2.3 (1.1-4.5) |
| ADAMTS13 ≤ p10 | OC use | 29 | 19 | 5.8 (2.7-12.4) | 5.1 (2.4-11.2) | 15 | 3.0 (1.4-6.2) | 2.7 (1.3-5.7) |
| Exposure 1 . | Exposure 2 . | Controls, n . | IS . | MI . | ||||
|---|---|---|---|---|---|---|---|---|
| Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | Cases, n . | OR1 (95% CI) . | OR2 (95% CI) . | |||
| VWF < p90 | ADAMTS13 > p10 | 507 | 107 | 1 (ref) | 1 (ref) | 130 | 1 (ref) | 1 (ref) |
| VWF < p90 | ADAMTS13 ≤ p10 | 57 | 20 | 2.2 (1.2-4.1) | 2.0 (1.1-3.8) | 21 | 1.6 (0.9-2.8) | 1.4 (0.8-2.5) |
| VWF ≥ p90 | ADAMTS13 > p10 | 57 | 31 | 3.2 (1.8-5.5) | 3.1 (1.7-5.5) | 39 | 2.6 (1.6-4.2) | 2.6 (1.6-4.3) |
| VWF ≥ p90 | ADAMTS13 ≤ p10 | 5 | 9 | 6.9 (2.0-23.0) | 5.8 (1.7-20.2) | 12 | 11.3 (3.6-35.2) | 9.8 (3.1-30.9) |
| VWF < p90 | No OC use | 372 | 65 | 1 (ref) | 1 (ref) | 92 | 1 (ref) | 1 (ref) |
| VWF < p90 | OC use | 192 | 62 | 2.2 (1.3-3.5) | 2.0 (1.2-3.3) | 59 | 2.2 (1.5-3.4) | 2.1 (1.4-3.3) |
| VWF ≥ p90 | No OC use | 45 | 14 | 1.8 (0.9-3.8) | 1.6 (0.8-3.5) | 29 | 2.7 (1.5-4.7) | 2.8 (1.5-5.0) |
| VWF ≥ p90 | OC use | 17 | 26 | 12.0 (5.5-26.2) | 11.4 (5.2-25.3) | 22 | 7.5 (3.6-15.7) | 7.1 (3.3-15.3) |
| ADAMTS13 > p10 | No OC use | 384 | 69 | 1 (ref) | 1 (ref) | 103 | 1 (ref) | 1 (ref) |
| ADAMTS13 > p10 | OC use | 180 | 69 | 2.6 (1.7-4.3) | 2.6 (1.6-4.1) | 66 | 2.6 (1.7-3.9) | 2.5 (1.6-3.8) |
| ADAMTS13 ≤ p10 | No OC use | 33 | 10 | 2.0 (0.9-4.6) | 1.8 (0.8-4.3) | 18 | 2.5 (1.3-4.9) | 2.3 (1.1-4.5) |
| ADAMTS13 ≤ p10 | OC use | 29 | 19 | 5.8 (2.7-12.4) | 5.1 (2.4-11.2) | 15 | 3.0 (1.4-6.2) | 2.7 (1.3-5.7) |
ORs were calculated by logistic regression and were adjusted for age, year of event/index year, and area of residence. OR2 values were also adjusted for hypercholesterolemia, hypertension, diabetes mellitus, and smoking.
ref indicates reference.
The use of OCs is known to be a risk factor for IS and MI, conferring approximately a 2-fold increased risk in young women.32,33 We therefore evaluated whether this risk was further increased by the concomitant presence of high VWF or low ADAMTS13. Strikingly, interaction analyses (Table 3) showed that high levels of VWF plasma levels and OC use yielded an OR1 of 12.0 (95% CI, 5.5-26.2) for IS and an OR1 of 7.5 (95% CI, 3.6-15.7) for MI. Similar results were obtained after adjustment for potential confounders (see OR2). Women on OCs with high levels of VWF were therefore > 5 times more likely to develop IS and > 3 times more likely to develop MI than women on OCs with lower VWF antigen levels. Joint analysis of OC use and ADAMTS13 Ag levels (Table 3) showed that the risk of IS and MI conferred by low ADAMTS13 Ag levels in women on OCs was 5.8 (95% CI, 2.7-12.4) and 3.0 (95% CI, 1.4-6.2), respectively. The OR2 decreased slightly after adjustment. A joint effect of low ADAMTS13 Ag levels and use of OCs was therefore observed for IS but not for MI.
Discussion
In the present study, we measured plasma VWF and ADAMTS13 antigen levels in 167 young women with IS, 202 young women with MI, and in 626 frequency-matched healthy controls.
Quartile analysis showed a concentration-dependent association of VWF and ADAMTS13 levels with the risk of IS and MI. The highest quartile of VWF conferred an OR2 of 6.7 (95% CI, 3.2-13.8) for IS and 4.2 (95% CI, 2.2-8.0) for MI. The lowest quartile of ADAMTS13 conferred an OR2 of 3.1 (95% CI, 1.6-5.8) for IS and 1.4 (95% CI, 0.8-2.4) for MI. The risks of IS and MI were also evident in both OR models when using p90 and p10 as cutoff values for VWF and ADAMTS13, respectively.
Interestingly, as can be seen from the interaction analyses, risks of IS and MI were conferred both by high VWF and low ADAMTS13, even when mutually adjusted, which raises an important point when considering the relationship between VWF and ADAMTS13 plasma levels. Although ADAMTS13 regulates VWF multimeric size through proteolysis, we and others have found no association between ADAMTS13 antigen levels and VWF antigen levels.24,25,31 Whereas for ADAMTS13, it is reasonable to assume that antigen and activity levels are essentially synonymous in healthy volunteers and in patients with CVD,35,36 this is not the case when comparing VWF antigen and function. One hypothesis is that the influence of ADAMTS13 concentration on the risk of IS and MI is manifest through the reduction of the multimeric size of circulating VWF or the modulation of VWF function locally at the site of vessel damage.
Joint analysis showed that having both high VWF and low ADAMTS13 increased the ORs 2- to 3-fold for IS and 4- to 7-fold for MI compared with having only one risk factor. The joint effect of high VWF and low ADAMTS13 was higher for MI (OR2 9.8) than for IS (OR2 5.8). However, because of the low number of individuals in this category the CIs were quite wide. Results from the joint analysis suggest that both VWF and ADAMTS13 are risk factors for IS and MI. In addition, the joint effect of high VWF and low ADAMTS13 supports previous findings suggesting that the ratio between VWF and ADAMTS13 in the same individual might be important.30,36,37
Our finding that the joint risk of having both high VWF and low ADAMTS13 is higher than that of the individual risk factor is in agreement with a former study investigating the joint risk of VWF and ADAMTS13 in a broad group including a combination of patients with coronary heart disease, IS, and peripheral arterial disease.31 Our study differs from the previous one because it only includes young women and involves cases that are more precise in phenotype (either IS or MI) and therefore also more precise in the respective effect estimates. Other case-control studies have investigated the individual risks conferred by VWF or ADAMTS13 antigen levels. Our results showing that VWF is a risk factor for IS and MI are in agreement with previous studies.21-27 Our results showing that low ADAMTS13 levels are associated with MI is in agreement with the findings from the GLAMIS case-control study.25 Others did not find an association between low ADAMTS13 and IS and MI, whereas one group found a positive association between ADAMTS13 plasma levels and MI.24,26,31 The reason for the discrepancy with the latter finding is not known. Whereas our study shows an association of low ADAMTS13 plasma levels with IS and MI, CIs were sometimes wide after stratification. Because the association between low ADAMTS13 and the risk of IS and MI is relatively weak, it is possible that previous studies have failed to show an association because of a lack of power.
Our results are consistent with recent in vivo studies in mice. It has been shown that VWF+/− and VWF−/− mice have a reduction in infarct volume of approximately 40% and 50%-60%, respectively, compared with wild-type (WT) mice after the induction of IS.38-40 The susceptibility of VWF−/− mice to IS was, however, restored after reconstitution of plasma VWF.38,39 These studies suggest that the lack of VWF or low VWF levels protects against IS, and that VWF plasma levels (and not VWF from endothelial cells or platelets) are important in the development of IS in mice.38,39 In contrast, it has been found that ADAMTS13−/− mice were susceptible to larger infarctions than WT mice.40,41 Interestingly, infusion of ADAMTS13 into WT mice after ischemic occlusion reduced the infarct volume by approximately 30%.40 Mice deficient in both ADAMTS13 and VWF had a phenotype similar to mice deficient in only VWF, showing that the action of ADAMTS13 is dependent on the presence of VWF.40
By stratifying data according to OC use, we showed that high VWF or low ADAMTS13 is associated with the risk of MI irrespective of OC use; however, CIs were wide after stratification. This was likely because of a loss in power, because only a small number of cases (14 and 10, respectively) were present in these strata. Joint analysis showed that patients with high levels of VWF who were on OCs had an approximately 11-fold higher risk of developing IS and an approximately 7-fold higher risk of developing MI than individuals with neither risk factor. Individuals with low ADAMTS13 using OCs were approximately 5 times more likely to develop IS than individuals with none of the risk factors. However, the risk of MI in patients with low ADAMTS13 antigen levels was not increased by the use of OCs.
Our study has some limitations because of its design. Blood was drawn after the event and only in cases that were not fatal. Because blood was drawn long after the event (median, > 69 months), it is unlikely that the antigen levels measured were influenced by the acute phase of the event. We cannot, however, exclude any chronic effect or account for potential reductions in exposure to environmental or behavioral risks after the event. Whereas VWF levels may be a result of vessel wall damage, this can even be the case in prospective studies. Association of genetic variations of VWF and ADAMTS13 with IS and MI might provide a means of excluding confounding and reverse causation. Preliminary support for associations between single nucleotide polymorphisms of VWF and ADAMTS13 and the risk of CVD has been provided by a few studies.42-44 However, large prospective studies evaluating single nucleotide polymorphisms will be required to establish the roles of VWF and ADAMTS13 in arterial thrombotic disease.44
The present study is the first, to our knowledge, to examine the risk of IS and MI conferred by the combination of both high VWF and low ADAMTS13 antigen levels in young women. We believe that analysis in a young population is of interest because they have been exposed for a shorter time to cardiovascular risk factors than older individuals. Similarly, atherosclerosis is likely to play a smaller role in young individuals. We have shown here that in young women, high VWF and low ADAMTS13 plasma levels are both associated with an increased risk of IS and MI, and that the use of OCs can further increase these risks.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in part in abstract form at the International Society on Thrombosis and Haemostasis, July 25, 2011, Kyoto, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to the participants of the RATIO case-control study and to the hospital and research staff involved in the study.
This work was supported by the British Heart Foundation (grant PG/07/005), the Netherlands Heart Foundation (grants 1997.063, 2001.069, and 2005B060), and the Prevention Fund, The Netherlands (grant 28-2879).
Authorship
Contribution: H.M.A., B.S., B.M.L., J.T.B.C., A.A., D.A.L., and F.R.R designed the research; H.M.A performed the experiments; and all authors analyzed the results and wrote the manuscripts.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helena Andersson, Centre for Haematology, Faculty of Medicine, Imperial College London, 5th Floor Commonwealth Bldg, Hammersmith Hospital Campus, Du Cane Road, W12 0NN, London, United Kingdom; e-mail: helena.andersson06@imperial.ac.uk.