Abstract
Angiogenesis requires integration of cues from growth factors, extracellular matrix (ECM) proteins, and their receptors in endothelial cells. In the present study, we show that the adaptor protein Shc is required for angiogenesis in zebrafish, mice, and cell-culture models. Shc knockdown zebrafish embryos show defects in intersegmental vessel sprouting in the trunk. Shc flox/flox; Tie2-Cre mice display reduced angiogenesis in the retinal neovascularization model and in response to VEGF in the Matrigel plug assay in vivo. Functional studies reveal a model in which Shc is required for integrin-mediated spreading and migration specifically on fibronectin, as well as endothelial cell survival in response to VEGF. Mechanistically, Shc is required for activation of the Akt pathway downstream of both integrin and VEGF signaling, as well as for integration of signals from these 2 receptors when cells are grown on fibronectin. Therefore, we have identified a unique mechanism in which signals from 2 critical angiogenic signaling axes, integrins and VEGFR-2, converge at Shc to regulate postnatal angiogenesis.
Introduction
Angiogenesis, the sprouting and growth of new blood vessels from preexisting vasculature, is critical for wound healing and in diseases such as rheumatoid arthritis, diabetes, and cancer.1 Angiogenesis is a highly coordinated tissue-remodeling process activated by proangiogenic growth factors such as VEGF, the expression of which is up-regulated in hypoxic or cancer cells. VEGF receptors expressed on the endothelial cell (EC) surface become activated when bound to the VEGF ligand, initiating signaling cascades that lead to EC proliferation, migration, survival, and tube formation.2 Basement membrane deposition and mechanical cues from the ECM transmitted via integrins also participate to coordinate vessel sprouting and remodeling in conjunction with the VEGF signaling pathway.3 Given their transmembrane structure, ability to form associations with adaptor molecules, and ability to bind to extracellular ligands, VEGF receptors and integrins are well positioned to serve as functional hubs during the angiogenic process.4
Adaptor proteins, which have no catalytic activity but instead promote protein-protein interactions, are important regulators of signaling pathways downstream of activated cell-surface receptors.5 The prototypical adaptor protein Shc is an evolutionarily conserved, ubiquitously expressed protein that was originally described as an oncogene because of its participation in the activation of Ras and MAPKs downstream of a multitude of receptors for various growth factors, cytokines, and hormones.6,7 Shc is expressed as 3 isoforms of 46, 52, and 66 kDa, all of which are products of the same gene, Shc1.8,9 Global knockout of Shc1 in mice causes embryonic lethality at embryonic day 11.5.10 These embryos exhibit severe defects in the cardiovascular system, including defective heart development and vessel remodeling. More detailed gene-targeting work has shown that the expression of the PTB domain of Shc specifically in cardiomyocytes is critical for midgestational heart development and embryonic life.11 Conditional knockout strategies have shown that Shc is also important for the proper development/function of other organs such as skeletal muscle,11 brain,12 cardiomyocytes,13 and thymocytes,14 because tissue-specific deletion of Shc resulted in living but underdeveloped mice. To address the role of Shc in angiogenesis in vivo, we studied loss of Shc function using morpholino (MO) antisense technology in zebrafish. In addition, we used the Tie2-Cre transgene to generate mice null for Shc in ECs and some hematopoietic cells.15 Surprisingly, these mice survived through development, enabling us to investigate the role of Shc in postnatal angiogenesis. We show herein that Shc is required for proper angiogenesis in vivo in both the zebrafish and mouse. Mechanistically, Shc is required for transmitting signals downstream of 2 major angiogenic signaling hubs, VEGFR-2 and integrins.
Methods
Zebrafish MO injection
Two splice-blocking MOs targeting the zebrafish ortholog of Shc1 (accession number LOC563639) were designed by GeneTools. The MO sequences are: ShcMO1: 5′-TGAAATGAATTGAATCTTACCCTGA −3′ and ShcMO2: 5′-ATAAAGAATTGGAAACCTTTCTCCT −3′. ShcMO2 resulted in better Shc knock-down and was used for the experiments. Shc or standard control MOs were injected into 1-cell-stage Tg(kdrl:egfp) zebrafish embryos at 8 ng (2×) or 16 ng (4×) per embryo. Embryos were scored and imaged at the 30-hour postfertilization stage by embedding in 1% agarose solution with 0.016% Tricaine to inhibit movement. Z-stacks were taken using 5× and 20× objectives on a Zeiss Pascal confocal microscope. For control 4× MO, n = 118; Shc 2× MO, n = 72; and Shc 4× MO, n = 105 embryos. Numbers indicate all fish counted from 3 independent experiments.
Mice
Shc floxed mice were a kind gift from Dr Kodi Ravichandran at the University of Virginia (Charlottesville, VA).16 Tie2-Cre [B6.Cg-Tg(Tek-cre)12Flv/J] and R26R [B6.129S4-Gt(ROSA)26Sortm1Sor/J] mice were purchased from The Jackson Laboratory. All housing, breeding, and experimental procedures using mice were in accordance with national guidelines and regulations and were approved by the Institutional Animal Care and Use Committee at the University of North Carolina–Chapel Hill.
X-Gal staining of R26R tissue
The Rosa26 reporter mice (The Jackson Laboratory) were used to monitor expression of Tie2-Cre. Male Shcflox/flox;Tie2-Cre+ were mated with female R26R mice to produce Shcflox/+;R26R+;Tie2-Cre+ and Shcflox/+;R26R+ offspring. Four-week-old mice were euthanized and tissues were quickly frozen and sectioned into 5-μm slices. Frozen sections were fixed in 0.2% glutaraldehyde and stained overnight with 1 mg/mL of X-Gal and then counterstained with Nuclear Fast Red. Slides were imaged using the 4×, 10×, and 40× objectives on an Olympus BX61 light microscope. Representative images are shown.
Matrigel plug assay
Four- to 6-week-old littermates were used to assay angiogenesis from subcutaneous tissue into Growth Factor Reduced Matrigel (BD Biosciences). Cold Matrigel was mixed with heparin (50 U/mL; Sigma-Aldrich) and VEGF (250 ng/mL) or vehicle (ddH2O). Mice were lightly anesthetized using isoflurane and cold Matrigel (0.5 mL) was injected into the abdominal subcutaneous tissue along the peritoneal midline. After 7 days, mice were euthanized and Matrigel plugs were removed and fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and H&E stained. Blood vessel infiltration into the plug was quantified in 4 sections per plug, each 100 μm apart, by counting number of cells per square millimeter using ImageJ Version 1.43 software. Images were obtained on an Olympus BX61 light microscope and data are expressed as mean values of 5-6 mice per condition.
MLEC isolation
Mouse lung ECs (MLECs) were isolated from 6- to 9-day-old Shcflox/flox and Shcflox/flox;Tie2-Cre+ littermate mice. Lungs were dissected from the mice and gently minced, collagenase digested, triturated, and strained. The resulting single-cell suspension underwent positive-cell selection using rat anti–mouse PECAM-1 Ab (BD Pharmingen)–conjugated magnetic Dynabeads (Invitrogen). PECAM+ cells were plated in tissue-culture flasks coated with 10 μg/mL of fibronectin (FN) and cultured in EGM-2 (Lonza). ECs were immortalized by transduction with polyoma middle T-antigen–expressing retrovirus and selected using G418. Four clones per genotype were isolated and validated for expression of the endothelial markers VEGFR2, VE-cadherin, and PECAM-1. Cells were cultured in EGM-2 (10% FBS).
Fibrin gel bead assay
Fibrin gel bead assays were performed following the protocol described previously.17 In short, MLECs were incubated with dextran-coated Cytodex 3 microcarriers (Amersham Pharmacia Biotech) at a concentration of 400 cells per bead in 1.5 mL of EGM-2 medium (Lonza) for 4 hours at 37°C. The following day, cell-coated beads were washed with EGM-2 and resuspended in 2 mg/mL of fibrinogen (Sigma-Aldrich) solution plus 0.15 U/mL of aprotinin (Sigma-Aldrich) at a concentration of 500 cell-coated beads/mL in 2.5 mg/mL. Next, 500 μL of fibrinogen/bead solution was added to 0.625 units of thrombin (Sigma-Aldrich) per well of a glass-bottom 24-well tissue-culture plate. Cultures were grown for 3 days in EGM-2 (10% FBS) and fixed in 4% paraformaldehyde on day 3. Cells were stained with phalloidin (FITC) to visualize actin and with DRAQ5 to mark EC nuclei and imaged using an Olympus FLV500 inverted confocal microscope using the 10× and 40× objectives. Sprouts were quantitated by counting the number of sprouts per bead and the number of nuclei per sprout. Sprouts were defined as protrusions containing 2 or more nuclei and > 15 beads were counted per genotype.
EC culture and lentivirus infection
Human umbilical vein ECs (HUVECs) were purchased from Lonza and maintained in M199 medium supplemented with 10% FBS, 30 μg/mL of EC growth supplement, 100 μg/mL of heparin, and 1× Pen/Strep (all from Sigma-Aldrich). HUVECs were starved in M199 medium with 0.5% FBS and 1× Pen/Strep for 4 hours before use in experiments unless otherwise noted. HUVECs were used between passage 2 and 9.
shRNAs against Shc (shShc) or nonspecific control (shNS) were designed by Dharmacon and subcloned into pLentiLox 5.0 vector for production of lentivirus in 293T cells, as described previously.18 shShc target sequence GGGGAGGAGTAACCTGAAA targets all 3 isoforms of the human Shc1 gene. The control shNS sequence GATCGACTTACGACGTTAT has no match in the human, mouse, or rat genomes.
Cell-spreading assay
HUVECs were detached with trypsin, washed with PBS, and kept in suspension for 30 minutes in the starvation medium. An equal number of cells were plated in starvation medium on glass coverslips coated in 10 μg/mL of FN, 10 μg/mL of collagen (CL; Millipore) or PBS. After 25 minutes, cells were fixed with 2% formaldehyde, permeabilized with 0.2% Triton X-100, and stained with Alexa Fluor 568–conjugated phalloidin to mark actin. Fluorescent images of randomly selected fields were acquired using a Zeiss Axiovert S100 microscope, and spread area was measured using the threshold function of ImageJ software. Values shown are means ± SEM (n = 2 independent experiments, > 100 cells counted per condition per experiment).
Cell-migration assays
Haptotaxis and chemotaxis to VEGF assays were performed using Boyden Chambers (Transwell; Corning) with 8-μm pores. For haptotaxis experiments, the underside of the Transwell filter was coated in either 10 μg/mL of FN or CL or PBS for 2 hours at 37°C.
For chemotaxis experiments, both the tops and the bottoms of the filters were coated with 10 μg/mL of FN for 2 hours at 37°C. Filters were washed in PBS and then blocked in PBS plus 3% BSA for 1 hour at 37°C and then washed again. HUVECs were FACS-sorted to obtain a pure population of green fluorescent–positive cells, and 10 000 cells were loaded into the upper portion of each chamber. After 4 hours of incubation, filters were washed 2 times in cold PBS and nonmigrating cells were removed from the tops of the chambers with a cotton swab. Filters were fixed in 2% formaldehyde and migration was quantitated by blind counting the number of migratory cells on the lower surfaces of the membranes using an inverted Zeiss Axiovert S100 microscope. Five random fields were imaged per filter. Values shown are means ± SEM (n = 3 independent experiments, 2 filters per condition per experiment).
EC survival assay
Lentivirus-infected HUVECs were seeded on FN- or CL-coated dishes and cultured in full HUVEC medium for 1 day. ECs were starved for 24 hours in starvation medium supplemented with 100 ng/mL of VEGF (Millipore) or vehicle control to induce apoptosis. After 24 hours, the medium was removed and cells were washed once with PBS to remove floating cell debris and lysed for Western blot. Values shown are means ± SEM (n = 4 independent experiments)
Retinal EC proliferation assays
Proliferating cells were marked using the Click-iT 5-ethynyl-2′-deoxyuridine (EdU) kit (Invitrogen) following the manufacturer's instructions, and 100 mg/g of EdU was injected intraperitoneally into each P5 pup. Two hours later, pups were anesthetized with isoflurane and euthanized by injection of 2% paraformaldehyde into the left ventricle to fix vessels. Retinas were dissected out and fixed in 4% paraformaldehyde and then stained with isolectin B4 (Sigma-Aldrich) to mark ECs, DAPI to mark nuclei, and EdU to mark proliferative nuclei. Flat-mounted retinas were imaged using a Zeiss LSM 710 confocal microscope and 20× and 60× objectives. Images were analyzed using ImageJ to count either number of branch points per 100 square micrometers or the percentage of proliferative nuclei by dividing the number of EdU-positive, isolectin-positive nuclei by the total number of isolectin-positive nuclei. For branch-point analysis, n = 17 Shcflox/flox mice and n = 15 Shcflox/flox;Tie2-Cre+ mice were used; for proliferation analysis, n = 5 Shcflox/flox mice and n = 8 Shcflox/flox;Tie2-Cre mice were used.
EC-adhesion assay
Lentivirus-infected HUVECs were starved for 4 hours and then removed from the dishes using 0.05% Trypsin-EDTA (Akt activation) or 20mM EDTA (ERK activation), spun down to pelleted cells, and resuspended in HUVEC starvation medium. An equal number of cells were seeded on dishes coated in FN or CL (10 μg/mL) and allowed to adhere for 15 minutes (ERK activation) or 30 minutes (Akt activation) in an incubator. Nonadherent cells were washed away with PBS and adherent cells were lysed and processed for Western blot analysis.
VEGF treatments and Western blot analysis
Lentivirus-infected HUVECs were grown to confluence on FN-coated dishes and starved for 4 hours. Medium was removed from all dishes and replaced with fresh starvation medium supplemented with 100 ng/mL of VEGF (Millipore) or vehicle control. Cells were incubated for 5 minutes, washed once in PBS, and lysed in buffer containing 1% Nonidet P-40, 150mM NaCl, 50mM Tris-HCl (pH 7.8), 2mM EDTA, 10mM NaF, 10mM Na2P2O7, 2mM Na3VO4 10 μg/mL of leupeptin, 4 μg/mL of pepstatin, and 0.1 U/mL of aprotinin. Lysates were cleared by spinning at maximum speed in a tabletop centrifuge and supernatant was combined with 10× Laemmli sample buffer and boiled briefly. Lysate was loaded into a 4%-12% NuPAGE Bis-Tris gel and run according to manufacturer's instructions using the LICOR Odyssey system.
Zebrafish were lysed in buffer containing 1% Nonidet P-40, 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 mM EGTA, 0.25% deoxycholate, 10 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 10 μg/mL of leupeptin, and 0.1 U/mL of aprotinin. Fifteen fish per condition were homogenized in a tissue tearer and lysate was cleared as described for HUVECs.
Abs used were: α-Shc (BD Biosciences), α-GAPDH (Millipore), and α-cleaved caspase 3 (Cell Signaling Technology). The intensity of the bands was quantified using ImageJ software, and the protein intensity was divided by GAPDH to normalize for total protein concentration. Quantification is shown as the mean of > 3 experiments.
Quantification and statistical analysis
Band intensity of immunoblots was quantified using ImageJ software. Each experimental group was analyzed using single-factor ANOVA. P values were obtained by performing the 2-tailed Student t test and using Excel 2003 software. Statistical significance was defined as P < .05.
Results
Angiogenesis in zebrafish requires Shc
A role for Shc in patterning of the vascular system and sprouting angiogenesis was assayed by depleting Shc protein from zebrafish embryos. Sprouting of intersegmental vessels (ISVs) dorsally from the aorta is a VEGF-driven process that can easily be visualized in situ using transgenic zebrafish.19 Shc protein was depleted from Tg(kdrl:egfp) zebrafish embryos at the 1-cell stage using a splice-site blocking MO targeted against Shc (supplemental Figure 2A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Shc-MO did not induce zebrafish death, suggesting that Shc is not required for zebrafish embryonic development during the first 3 days after fertilization. Interestingly, global Shc depletion in the zebrafish embryo caused angiogenesis defects specifically, whereas all other tissues appeared normal. Shc morphants showed impaired ISV formation at 30 hours after fertilization (Figure 1A). At both a high (16 ng/embryo) and a low (8 ng/embryo) concentration, 73% and 55% of Shc-MO zebrafish displayed a cardiovascular defect, compared with only 12% of zebrafish injected with a high concentration of standard control-MO (Figure 1B). The predominant vascular phenotype observed was defective growth of ISVs dorsally and improper dorsal longitudinal anastomotic vessel formation, and other defects ranged from complete loss of ISV sprouts or abnormal overall ISV patterning (termed “severe CV defects”) to dilated caudal ventral vein and partial connection of ISVs to the dorsal longitudinal anastomotic vessel (“mild CV defects”). No obvious defects were observed in heartbeat, aorta morphology, or overall zebrafish patterning, indicating a specific role for Shc in angiogenesis during zebrafish development. These results are consistent with the phenotype of the global Shc-knockout mouse, which exhibited cardiovascular development defects.10 In contrast to the mouse, Shc-MO zebrafish did not display increased lethality compared with control-MO–injected fish. This apparent discrepancy may be due to the unique ability of zebrafish to survive significantly longer than mice without a functional heart or vascular system20 and/or the incomplete depletion of Shc protein in the Shc-MO zebrafish.
Shc is required for intersegmental vessel sprouting angiogenesis in zebrafish. (A) Shc protein depletion in Tg(kdrl:egfp) zebrafish embryos results in defective angiogenesis 30 hours after fertilization. Representative images of trunk vasculature are shown, with anterior on the left. Shc-MO fish exhibited a range of vascular phenotypes, the most common being delayed or defective intersomitic vessel sprouting and growth dorsally from the aorta. Scale bars indicate 200 μm. (B) Quantification of phenotypes observed in all living fish at 30 hours after fertilization displayed as a percentage of total. Control 4× MO, n = 118; Shc 2× MO, n = 72; and Shc 4× MO, n = 105 embryos. Numbers indicate all fish counted from 3 independent experiments.
Shc is required for intersegmental vessel sprouting angiogenesis in zebrafish. (A) Shc protein depletion in Tg(kdrl:egfp) zebrafish embryos results in defective angiogenesis 30 hours after fertilization. Representative images of trunk vasculature are shown, with anterior on the left. Shc-MO fish exhibited a range of vascular phenotypes, the most common being delayed or defective intersomitic vessel sprouting and growth dorsally from the aorta. Scale bars indicate 200 μm. (B) Quantification of phenotypes observed in all living fish at 30 hours after fertilization displayed as a percentage of total. Control 4× MO, n = 118; Shc 2× MO, n = 72; and Shc 4× MO, n = 105 embryos. Numbers indicate all fish counted from 3 independent experiments.
Endothelial Shc is required for proper angiogenesis in vivo
To specifically inactivate the Shc1 gene in ECs, female mice carrying floxed alleles of Shc exons 1 and 216 were intercrossed with male transgenic mice expressing Cre recombinase under the control of the Tie2 promoter, which is expressed specifically in ECs and some hematopoietic cells.15 In this cross, the Tie2-Cre allele was always donated from the father to minimize leakage of Cre expression into other tissues, which can occur when Tie2-Cre is donated by the mother. Surprisingly, Shcflox/flox;Tie2-Cre+ mice were born at the expected Mendelian ratio and these animals display no gross anatomic abnormalities or decrease in fertility compared with Shcflox/flox controls. To verify tissue-specific Cre/loxP recombination in our mice, we crossed Shcflox/flox;Tie2-Cre+ mice to mice that carry the Rosa26 Lac-Z reporter allele. X-Gal staining of the carotid artery, heart, and retina was restricted to the endothelium (supplemental Figure 1). Cre expression was not mosaic, because X-Gal staining was seen in nearly all ECs. To confirm that the Shc protein was reduced in ECs, primary lung ECs (MLECs) were isolated from Shcflox/flox;Tie2-Cre+ and Shcflox/flox littermates. Western blot analysis revealed a complete reduction in all 3 Shc isoforms in the Shcflox/flox;Tie2-Cre+ mice (supplemental Figure 3A).
To determine whether angiogenesis is affected in Shcflox/flox;Tie2-Cre+ mice, we used 2 in vivo models: neonatal retinal neovascularization and the Matrigel plug assay. Vascularization of the murine retina commences after birth, as the vessels originating at the optic nerve spread radially over the inner surface of the retina on the preexisting template of astrocytes, guided by a gradient of VEGF-A to form a 2D vascular plexus.21 At postnatal day 5, retinas were isolated and stained with isolectin B4 to mark ECs. Shcflox/flox;Tie2-Cre+ mice exhibited a less dense primitive plexus at the vascular front compared with both Shcflox/flox and Shc wt/wt;Tie2-Cre+ controls (Figure 2A). Vascular density in the retina was quantified by counting the number of branch points per 100 square micrometer area and the percentage vascular area, both of which revealed a significant decrease in Shcflox/flox;Tie2-Cre+ compared with littermate controls (Figure 2A bottom). Both genotypes of control mice, Shcflox/flox and Shc wt/wt;Tie2-Cre+, showed equal retinal vascular density, indicating that Tie2-Cre expression itself is not responsible for the phenotype. Therefore, only Shcflox/flox littermate controls were used in the remaining experiments.
Endothelial Shc knockout causes defective angiogenesis in vivo. (A) Shc knockout in ECs results in decreased vascular density in the postnatal retina. Retinas from P5 mice were stained with isolectin-B4 Alexa Fluor 488 to visualize ECs. Vascular density was quantified by counting branch points per 100 square micrometers and percentage of vascular area (performed blind by 2 different people). Shcflox/flox, n = 17; Shcflox/flox;Tie2-Cre+, n = 15; and Shc wt/wt;;Tie2-Cre+, n = 9 mice. Scale bars indicate 100 μm. (B) Matrigel Plug assay in 4- to 6-week-old mice reveals a role for EC Shc in angiogenesis toward VEGF. Matrigel plugs containing 250 ng/mL of VEGF or vehicle alone were implanted into each mouse. After 7 days, plugs were H&E stained and microvessels per square millimeter were counted in serial sections through the plug. Values are means ± SEM (by Student t test). Shcflox/flox, n = 5 and Shcflox/flox;Tie2-Cre+, n = 6 mice.
Endothelial Shc knockout causes defective angiogenesis in vivo. (A) Shc knockout in ECs results in decreased vascular density in the postnatal retina. Retinas from P5 mice were stained with isolectin-B4 Alexa Fluor 488 to visualize ECs. Vascular density was quantified by counting branch points per 100 square micrometers and percentage of vascular area (performed blind by 2 different people). Shcflox/flox, n = 17; Shcflox/flox;Tie2-Cre+, n = 15; and Shc wt/wt;;Tie2-Cre+, n = 9 mice. Scale bars indicate 100 μm. (B) Matrigel Plug assay in 4- to 6-week-old mice reveals a role for EC Shc in angiogenesis toward VEGF. Matrigel plugs containing 250 ng/mL of VEGF or vehicle alone were implanted into each mouse. After 7 days, plugs were H&E stained and microvessels per square millimeter were counted in serial sections through the plug. Values are means ± SEM (by Student t test). Shcflox/flox, n = 5 and Shcflox/flox;Tie2-Cre+, n = 6 mice.
The Matrigel plug assay, in which microvessel growth is induced toward an angiogenic factor source (in this case, VEGF), adult mice were injected with 2 plugs each, one containing vehicle and the other supplemented with VEGF to induce angiogenesis into the plug. VEGF induced neovascularization of Matrigel implants in Shcflox/flox controls, whereas neovascularization was impaired in Shcflox/flox;Tie2-Cre+ littermates (Figure 2B). These data suggest that endothelial Shc is required for proper postnatal angiogenesis in vivo.
Endothelial Shc is required for tube assembly and sprouting in vitro
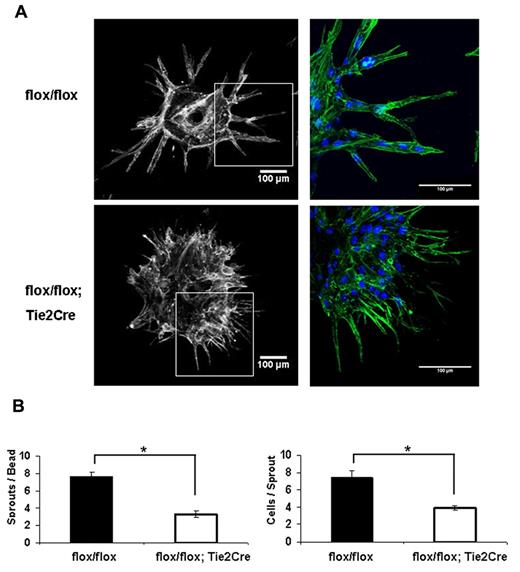
To further determine the role of Shc in the EC angiogenic response, we performed the fibrin gel bead assay. MLECs isolated from Shcflox/flox;Tie2-Cre+ and Shcflox/flox mice were coated on beads and embedded in fibrin gel. Control Shcflox/flox ECs sprouted outward off the bead and lumenized to form capillary-like vessels in the 3D fibrin matrix, as is typically seen using HUVEC. Interestingly, Shcflox/flox;Tie2-Cre+ MLECs displayed a striking defect in both the number and size of sprouts (Figure 3A). Whereas Shcflox/flox;Tie2-Cre+ MLECs were able to extend filopodia out into the matrix at a normal or even enhanced rate, these filopodia failed to develop into full sprouts and the tip cells remained stuck on the bead. Quantification revealed a significant reduction in number of sprouts per bead and in the number of cells per sprout, indicating an important role for Shc in EC sprouting (Figure 3B).
Endothelial Shc is required for tube assembly and sprouting in vivo. (A) The fibrin gel bead assay was performed using MLECs isolated from the same mice used in Figure 2. On day 3 after seeding the cell-covered beads in gel, cultures were fixed and stained for phalloidin (green) and DRAQ5 (blue). Quantifications (bottom) were performed by counting at least 15 beads per genotype and are expressed as means ± SEM of n = 2 independent experiments at 4 replicates per experiment. *P < .05.
Endothelial Shc is required for tube assembly and sprouting in vivo. (A) The fibrin gel bead assay was performed using MLECs isolated from the same mice used in Figure 2. On day 3 after seeding the cell-covered beads in gel, cultures were fixed and stained for phalloidin (green) and DRAQ5 (blue). Quantifications (bottom) were performed by counting at least 15 beads per genotype and are expressed as means ± SEM of n = 2 independent experiments at 4 replicates per experiment. *P < .05.
Shc is required for integrin-mediated EC signaling
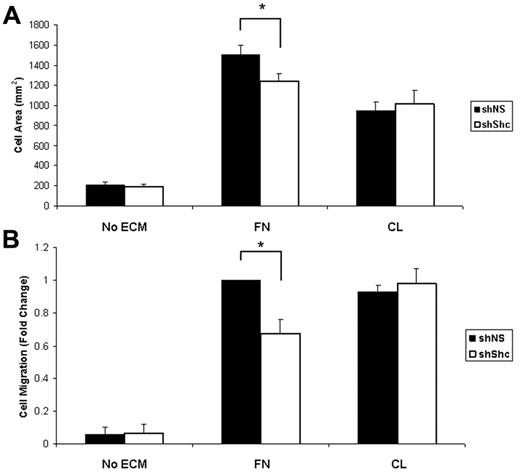
To understand the mechanism underlying the role for Shc in angiogenesis, we examined signaling downstream of 2 major angiogenic receptors, integrins and VEGFR2, in ECs. Previous work has shown that Shc binds to a subset of activated integrins and mediates signaling. After outside-in integrin activation by ligation to its ECM ligand, Shc is phosphorylated and recruited to integrins α5β1 (FN receptor) and αvβ3 (FN/vitronectin), but not to α2β1 (CL) or α6β1 (laminin).22 We therefore tested the role of Shc in integrin-dependent angiogenic responses. For the following experiments, HUVECs were infected with lentivirus that expresses either shShc or shNS to deplete the Shc protein (supplemental Figure 2B). The role of Shc in integrin-mediated cell spreading on ECM was tested by seeding equal numbers of ECs on FN or CL and measuring the cell area. Whereas there was no difference in spreading on CL in the absence of Shc, Shc-depleted ECs showed impaired spreading on FN compared with control ECs (Figure 4A), suggesting that Shc is required specifically for EC spreading on FN. To determine whether migration toward FN also requires Shc, we performed haptotaxis assays using Boyden chambers. Similar to the spreading experiments, EC migration toward FN was impaired in Shc-depleted ECs, whereas migration toward CL occurred independently of Shc (Figure 4B) These results indicate that Shc is required for integrin-mediated spreading and migration toward FN, suggesting that Shc selectively mediates angiogenic signaling downstream of FN-binding integrins.
Shc is required for integrin-mediated spreading and haptotaxis on FN but not CL. (A) Shc is required for cell spreading on FN. Equal numbers of lentivirus-infected HUVECs were seeded on coverslips coated with 10 μg/mL of FN, 10 μg/mL of CL, or vehicle (PBS) and allowed to spread for 25 minutes. The cell area was measured using ImageJ software. Values shown are means ± SEM (n = 2 independent experiments, > 100 cells counted per condition per experiment). (B) Haptotaxis was measured using Boyden chambers coated on the underside with 10 μg/mL of FN, 10 μg/mL of CL, or vehicle (PBS) and blocked with 3% BSA. Cells that had migrated to the underside of the chamber were counted using an inverted microscope. Five random fields were imaged per filter. Values shown are means ± SEM (n = 3 independent experiments, 2 filters per condition per experiment). *P < .05
Shc is required for integrin-mediated spreading and haptotaxis on FN but not CL. (A) Shc is required for cell spreading on FN. Equal numbers of lentivirus-infected HUVECs were seeded on coverslips coated with 10 μg/mL of FN, 10 μg/mL of CL, or vehicle (PBS) and allowed to spread for 25 minutes. The cell area was measured using ImageJ software. Values shown are means ± SEM (n = 2 independent experiments, > 100 cells counted per condition per experiment). (B) Haptotaxis was measured using Boyden chambers coated on the underside with 10 μg/mL of FN, 10 μg/mL of CL, or vehicle (PBS) and blocked with 3% BSA. Cells that had migrated to the underside of the chamber were counted using an inverted microscope. Five random fields were imaged per filter. Values shown are means ± SEM (n = 3 independent experiments, 2 filters per condition per experiment). *P < .05
Shc is required for VEGF-mediated EC signaling
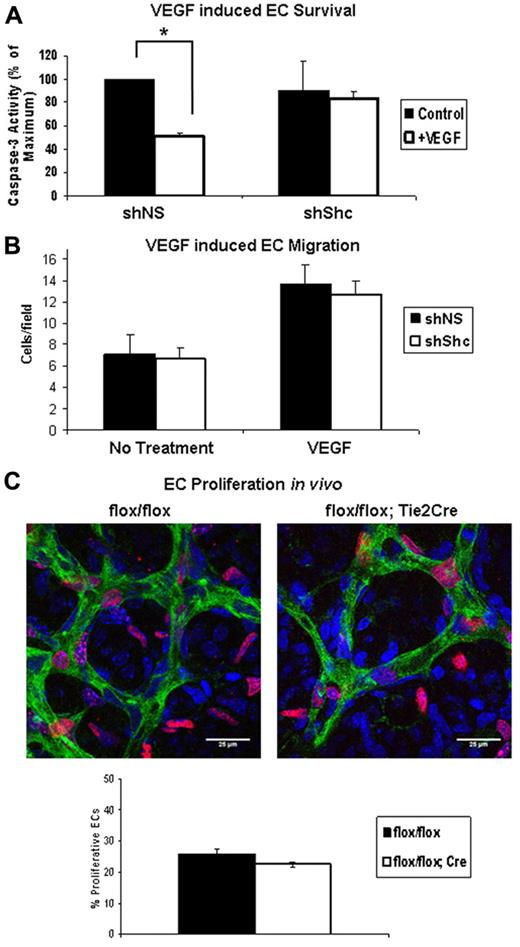
VEGF induces Shc phosphorylation and its association with VEGFR-2 and VE-cadherin.23,24 However, the role of Shc, if any, in signaling downstream of VEGF remains unexplored. We assayed the role of Shc in VEGF-induced EC survival and migration in vitro, as well as proliferation of retinal ECs in vivo. EC survival was assayed by inducing apoptosis in ECs in the presence or absence of VEGF. Apoptosis was quantified by measuring the level of cleaved caspase 3 (supplemental Figure 4A) present in the cell lysates. VEGF treatment resulted in a 50% decrease in cleaved caspase 3 in shNS control cells, whereas shShc ECs showed no significant protection from apoptosis (Figure 5A). To determine the role of Shc in VEGF-induced migration, we assayed chemotaxis toward a VEGF gradient. Baseline migration of shNS and shShc ECs was similar and, interestingly, migration toward VEGF was induced in both cell types, indicating that Shc is not required for EC migration toward a VEGF gradient (Figure 5B). In the developing retina, vessel outgrowth occurs by proliferation of ECs and migration of endothelial tip cells in response to the VEGF gradient released from the underlying astrocytes.25 To investigate whether Shc deficiency affects the proliferation rate of retinal ECs, we analyzed EdU incorporation into endothelial nuclei 2 hours after injection into the P5 mice (Figure 5C). Retinas were stained to mark ECs green (isolectin), all cell nuclei blue (DAPI), and proliferating cells red (EdU). By comparing the number of cells that stained positive for all 3 markers divided by the total number of ECs, we found that the number of EdU+ EC nuclei was slightly lower in Shcflox/flox;Tie2-Cre+ mice compared with controls (Figure 5C), but these differences did not reach statistical significance. Therefore, Shc does not play a significant role in EC proliferation. These data are consistent with a model in which Shc function is important for survival signaling in response to VEGF, but not for VEGF-induced migration or proliferation.
Shc is required for VEGF-induced EC survival but not migration toward VEGF or proliferation. (A) HUVECs were serum starved for 24 hours with or without 100 ng/mL of VEGF to induce apoptosis. Lysates were immunoblotted for cleaved caspase 3 and GAPDH as a loading control. Survival was quantified by comparing the amount of cleaved caspase 3 present in lysate. Values shown are means ± SEM (n = 4 independent experiments). (B) Chemotaxis toward the VEGF gradient was measured using Boyden chambers containing 100 ng/mL of VEGF or vehicle in the lower well. After 4 hours of migration, cells that had migrated to the underside of the membrane were counted using an inverted microscope. Five random fields were imaged per filter. Values shown are means ± SEM (n = 3 independent experiments, 2 filters per condition per experiment). *P < .05. (C) EC proliferation was assayed in the P5 mouse retina. EdU reagent was injected intraperitoneally and 2 hours later retinas were harvested. Retinas were stained with isolectin (green) to mark ECs, DAPI (blue) to mark all cell nuclei, and EdU (red) to mark proliferating nuclei. Proliferation of ECs was quantified by counting the number of isolectin/EdU+ nuclei and dividing by the number isolectin/DAPI+ nuclei. Values shown are means ± SEM (Shcflox/flox, n = 5 and Shcflox/flox;Tie2-Cre, n = 8).
Shc is required for VEGF-induced EC survival but not migration toward VEGF or proliferation. (A) HUVECs were serum starved for 24 hours with or without 100 ng/mL of VEGF to induce apoptosis. Lysates were immunoblotted for cleaved caspase 3 and GAPDH as a loading control. Survival was quantified by comparing the amount of cleaved caspase 3 present in lysate. Values shown are means ± SEM (n = 4 independent experiments). (B) Chemotaxis toward the VEGF gradient was measured using Boyden chambers containing 100 ng/mL of VEGF or vehicle in the lower well. After 4 hours of migration, cells that had migrated to the underside of the membrane were counted using an inverted microscope. Five random fields were imaged per filter. Values shown are means ± SEM (n = 3 independent experiments, 2 filters per condition per experiment). *P < .05. (C) EC proliferation was assayed in the P5 mouse retina. EdU reagent was injected intraperitoneally and 2 hours later retinas were harvested. Retinas were stained with isolectin (green) to mark ECs, DAPI (blue) to mark all cell nuclei, and EdU (red) to mark proliferating nuclei. Proliferation of ECs was quantified by counting the number of isolectin/EdU+ nuclei and dividing by the number isolectin/DAPI+ nuclei. Values shown are means ± SEM (Shcflox/flox, n = 5 and Shcflox/flox;Tie2-Cre, n = 8).
Integration of VEGF and integrin signaling via Shc
Our data show a role for Shc in processes downstream of both VEGF and integrins. We hypothesized that Shc mediates cross-talk between these 2 receptors. To test this hypothesis, we performed survival experiments on ECs plated on FN versus CL. VEGF treatment resulted in an approximately 50% decrease in apoptosis in shNS control cells grown on either FN or CL, similar to what was seen in Figure 5A. Interestingly, shShc ECs grown on CL showed similar VEGF-induced survival as control shNS cells, whereas shShc ECs grown on FN showed no significant protection from apoptosis (Figure 6). These data suggest that Shc integrates VEGF and integrin signals specifically on FN.
Survival requires integration of VEGF and integrin signaling through Shc. (A) HUVECs were seeded on FN- or CL-coated dishes, and then serum starved for 24 hours with or without 100 ng/mL of VEGF to induce apoptosis. Lysates were immunoblotted for cleaved caspase 3 and GAPDH as a loading control. Survival was quantified by comparing the amount of cleaved caspase 3 present in lysate. Values shown are means ± SEM (n = 3 independent experiments).
Survival requires integration of VEGF and integrin signaling through Shc. (A) HUVECs were seeded on FN- or CL-coated dishes, and then serum starved for 24 hours with or without 100 ng/mL of VEGF to induce apoptosis. Lysates were immunoblotted for cleaved caspase 3 and GAPDH as a loading control. Survival was quantified by comparing the amount of cleaved caspase 3 present in lysate. Values shown are means ± SEM (n = 3 independent experiments).
Shc mediates Akt activation downstream of VEGF and integrin activation
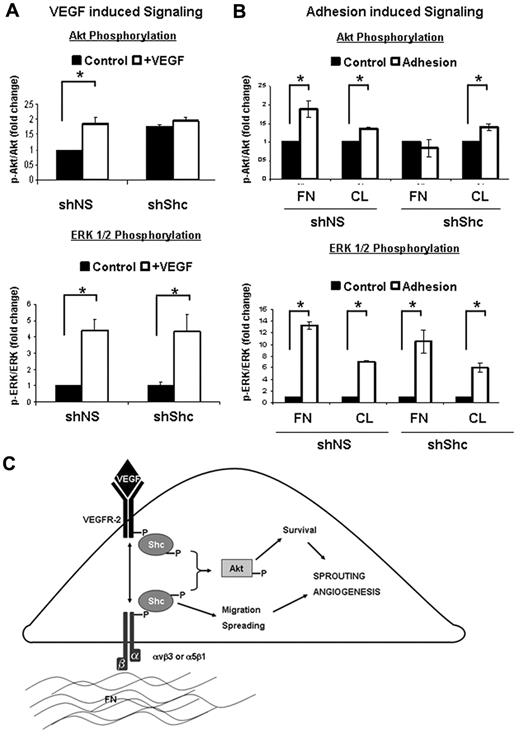
To further delineate the signaling pathways that are mediated by Shc downstream VEGF and integrin signaling, we assayed activation of 2 key signaling cascades, Akt and ERK1/2. shShc ECs treated with VEGF failed to activate Akt, whereas VEGF-induced ERK1/2 activation was similar to shNS control ECs (Figure 7A). Interestingly, the requirement for Shc in the activation of Akt was specific to VEGF, because Epidermal Growth Factor induced robust activation of Akt in both shNS and shShc ECs (supplemental Figure 4B). Similarly, Akt activation by adhesion of ECs to FN was impaired in shShc ECs, whereas shShc ECs plated on CL could activate Akt normally (Figure 7B), indicating that Shc mediates Akt activation specifically downstream of FN-binding integrins. In contrast, ERK1/2 was activated similarly in both shNS and shShc ECs on both FN and CL, indicating that Shc is not important for ERK1/2 activation downstream of either FN or CL binding integrins. These data are consistent with a model in which Shc function is important for Akt signaling, which promotes survival downstream of VEGF specifically on FN, whereas Shc is dispensable for ERK1/2 activation and EC proliferation.
Shc is required for specific signal transduction pathways downstream of integrins and VEGF. (A) The role of Shc in VEGF signaling was assayed in HUVECs. Cells were treated for 5 minutes with 100 ng/mL of VEGF or vehicle. Cell lysates were separated by SDS-PAGE and immunoblotted for the indicated proteins. Quantitation values shown are means ± SEM (n = 4 independent experiments). (B) The role of Shc in integrin signaling was assayed in HUVECs. Cells were allowed to adhere and spread on FN or CL (10 μg/mL) or kept as controls. Cell lysates were separated by SDS-PAGE and immunoblotted for the indicated proteins. Quantitation values shown are means ± SEM (n = 3 independent experiments). *P < .05. (C) Schematic model of how Shc is thought to regulate angiogenesis in ECs. Shc participates in signaling from FN-binding integrins such as αvβ3 and α5β1, which is required for EC spreading and migration. Shc is simultaneously required for EC survival induced by VEGF. Loss of Shc results in attenuation of Akt activation by the integrin and VEGF pathways in ECs and therefore results in defective angiogenesis, as is seen in the zebrafish and the mouse.
Shc is required for specific signal transduction pathways downstream of integrins and VEGF. (A) The role of Shc in VEGF signaling was assayed in HUVECs. Cells were treated for 5 minutes with 100 ng/mL of VEGF or vehicle. Cell lysates were separated by SDS-PAGE and immunoblotted for the indicated proteins. Quantitation values shown are means ± SEM (n = 4 independent experiments). (B) The role of Shc in integrin signaling was assayed in HUVECs. Cells were allowed to adhere and spread on FN or CL (10 μg/mL) or kept as controls. Cell lysates were separated by SDS-PAGE and immunoblotted for the indicated proteins. Quantitation values shown are means ± SEM (n = 3 independent experiments). *P < .05. (C) Schematic model of how Shc is thought to regulate angiogenesis in ECs. Shc participates in signaling from FN-binding integrins such as αvβ3 and α5β1, which is required for EC spreading and migration. Shc is simultaneously required for EC survival induced by VEGF. Loss of Shc results in attenuation of Akt activation by the integrin and VEGF pathways in ECs and therefore results in defective angiogenesis, as is seen in the zebrafish and the mouse.
Discussion
In this study, we present evidence that the adaptor protein Shc is required for postnatal angiogenesis in the zebrafish, mouse, and cell culture models. Shc morphant zebrafish embryos show defects in ISV sprouting in the trunk, whereas Shcflox/flox;Tie2-Cre+ mice display impaired angiogenesis in the retina and in the Matrigel plug assay in vivo. Using an in vitro model of angiogenesis, we have shown that Shc is required for sprouting and tube formation. Mechanistically, Shc integrates signals downstream of integrins and VEGF. Shc is required for integrin-mediated spreading and migration specifically on FN, as well as survival in response to VEGF. Shc integrates VEGF and integrin signaling, because VEGF-induced survival on FN requires Shc, whereas survival in ECs on CL does not. Activation of the Akt, but not the ERK1/2, pathway in response to both VEGF and integrin activation depends on Shc. Combined, these processes are critical for angiogenesis and provide a mechanism by which Shc integrates signals from VEGF and integrins to mediate angiogenesis (Figure 7C).
Given the large number of signaling networks that need to be organized and integrated for new vessels to form, signaling hubs may be important during angiogenesis.4 In this manner, both integrin and VEGF-receptor complexes represent central signaling axes during angiogenesis. Activation of either VEGFR-2 or αvβ3 induces physical association of the 2 receptors, which is important for VEGFR-2 phosphorylation.26 Function of both receptors is required for proper signaling, because inhibition of αvβ3 or VEGFR-2 function decreases VEGFR-2 activation and complex formation.27,28 The results of the present study show that Shc is required for mediating and integrating angiogenic responses downstream of both integrins (FN-binding integrins specifically) and VEGF, thus coordinating the angiogenic process as a whole.
Shc was originally described as an oncogene, and mutation of Shc attenuates tumor growth in mice.29 Shc overexpression in fibroblasts causes transformation9 and Shc is required for cellular transformation in ErbB2-overexpressing breast cancer cells,30 as well as in mammary tumors induced by polyoma middle T expression.31 In humans, clinical studies have associated Shc activation with poor patient prognosis.29 These data, combined with our current findings, suggest that Shc is critical for many steps of tumorigenesis, including cellular transformation of tumor cells themselves, as well as angiogenesis in ECs that feed the tumor and enable its growth. Therefore, Shc may be an interesting target for cancer treatment at multiple levels.
Expression of the PTB domain of Shc in cardiomyocytes is essential for embryonic heart development.11 Interestingly, mice with a conditional deletion of Shc in specific organs such as skeletal muscle,11 thymocytes,14 or brain12 live to adulthood, exhibiting defects only in the function of the tissue in which Shc was removed. Similarly, we now show that endothelial Shc expression is not required for embryonic development, but is required postnatally for angiogenesis. Induction of Tie2-Cre expression has been reported at embryonic day 9.5,15 which precedes embryonic lethality of the global Shc knockout at embryonic day 11.5, so mistiming of Shc gene excision does not appear to be the reason for Shcflox/flox;Tie2-Cre+ mouse survival. Emerging research has set a precedent for the idea that conditional gene knockout using the Tie2-Cre transgene can result in mice that initially develop a normal vasculature while exhibiting defective angiogenic capacity. Tie2-Cre–mediated conditional knockout of genes such as Endothelin-1,32,33 TFPI,34 ADAM17,35 PPARγ,36 and Dicer37 yield viable mice with cardiovascular defects, whereas the corresponding global knockout animal is embryonically lethal. Therefore, genes such as Shc and others appear to have differing roles in developmental versus postnatal angiogenesis. This hypothesis is strengthened in light of the literature on proteins that interact with Shc. Our results indicate that Shc is required for signaling downstream of FN-binding integrins such as αvβ3 and/or α5β1, which are up-regulated during angiogenesis. Surprisingly, endothelial knockout of αv,38 β3,39 or α540 results in viable mice, whereas antagonism of either of these integrins with blocking Abs causes a defect in angiogenesis.41-43 We have also shown herein that Shc mediates a subset of signaling responses downstream of VEGF. In particular, Shc is required for EC survival but not proliferation in response to VEGF.
We recently reported a role for Shc in mechanotransduction in response to shear stress.44 Hemodynamic forces are emerging as an important regulator of angiogenesis in some vascular beds such as the aortic arch45,46 and yolk sac47 in both the mouse and fish. Flow also promotes hematopoietic cell development48,49 in vivo and atheroprotective laminar flow inhibits HUVEC tubule formation and migration in vitro.50 Therefore, a role for Shc in flow-driven angiogenesis is an attractive idea. Integration of VEGF- and flow-dependent signaling was recently reported during zebrafish vascular remodeling,46 and future experiments are aimed at understanding the role of Shc in these processes. Angiogenesis involves a complex interplay of mechanical forces, ECM remodeling, and pro- and anti-angiogenic growth factors, all signaling simultaneously in ECs. Adaptor proteins such as Shc are likely to be responsible for the integration of these signals because of their ability to bind many receptors and are emerging as signaling nodes critical for many vascular processes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. S. Ravichandran for generous donation of Shc flox mice; J. C. Chappell for help with retina isolation; E. J. Flynn, M. J. Woolls, J. F. Rawls, and S. W. Jin of the UNC Zebrafish Core Facility for help with zebrafish experiments; K. McNaughton for histology support; and colleagues in the Tzima laboratory for critical reading of the manuscript.
This work was supported by the National Institutes of Health (grants T32 HL069768 to D.T.S., HL088632 to E.T., and R01 HL43174), and by the American Heart Association (grants 3490004 and 0635228N to E.T. and predoctoral fellowship 2230268 to D.T.S.). E.T. is an Ellison Medical Foundation New Scholar.
National Institutes of Health
Authorship
Contribution: D.T.S. designed and performed the research, analyzed the data, and wrote the manuscript; Z.C. and D.M.W. performed the experiments; V.L.B. provided the reagents; and E.T. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ellie Tzima, Dept of Cell Molecular Physiology, 6341C Medical Biomolecular Research Bldg, Campus Box 7545, 111 Mason Farm Rd, Chapel Hill, NC 27599; e-mail: etzima@med.unc.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal