Abstract
Pathologische anatomie leiden endothelium (PAL-E) antibody has been used for more than 20 years as a prototype marker for vascular endothelium. The elusive target of this antibody has been claimed to be plasmalemma vesicle-associated protein-1 (PV-1) and neuropilin-1 (NRP-1). Using immunofluorescence, we show that PAL-E, anti–PV-1, anti–NRP-1, and anti-CD31 antibodies show largely identical staining patterns in the vasculature of different tissues. However, PV-1–transfected cells only bind PAL-E and anti–PV-1 antibodies, whereas NRP-1 transfectants stain with anti–NRP-1 antibodies in flow cytometry. Using lysates from tissues and transfected cells, we further confirm that the molecule recognized by PAL-E and anti–PV-1 antibodies is not NRP-1 but PV-1. Nevertheless, coimmunoprecipitation studies unambiguously demonstrate that NRP-1 can form complexes with PV-1. This connects, for the first time, 2 molecules involved in leukocyte trafficking and angiogenesis, thereby opening interesting possibilities for future research in this field.
Introduction
The ability to discriminate between vessels of vascular and lymphatic origin is critical in many studies in the fields of immunology, vascular biology, pathology, and tumor biology. Several markers, including CD31, endoglin, factor VIII, and pathologische anatomie leiden-endothelium (PAL-E), have been used to identify vascular endothelium. Because of its specificity for endothelial cells of capillaries, venules, and medium-sized veins,1 the antibody PAL-E has been the gold standard among these markers.
Despite its widespread use, the identification of the PAL-E antigen has proven difficult, and there has been marked confusion regarding the identity of the molecule recognized by PAL-E.2-4 We identified the molecular target of PAL-E as plasmalemma vesicle-associated protein-1 (PV-1).3,5 Recently, this was challenged by reporting neuropilin-1 (NRP-1) as the antigen for PAL-E.4 PV-1 is a heavily glycosylated approximately 55- to 65-kDa glycoprotein originally discovered in rat lung endothelium and subsequently found in stomatal diaphragms of endothelial caveolae and transendothelial channels.6 NRP-1, on the other hand, was first found as a semaphorin receptor and neuronal cell guidance molecule7 and later also shown to function during angiogenesis as a VEGFR-2 coreceptor binding VEGF-A165.8,9 This study was undertaken to elucidate the relationship between the antibody PAL-E and the proteins PV-1 and NRP-1.
Methods
For detailed information, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The use of human material and all procedures were approved by the Ethical Board of Turku University Hospital and abided by the Declaration of Helsinki.
Immunostainings
Frozen human tissues were stained with primary antibodies. Isotype specific secondary antibodies coupled to either Alexa Fluor-488 or Alexa Fluor-546 (Invitrogen) were used. Samples were analyzed on a LSM 510 confocal microscope (Carl Zeiss Microimaging).
For flow cytometry, HEK EBNA cells were transiently transfected (Lipofectamine 2000; Invitrogen) with pcDNA3.1 plasmids containing PV-1, NRP-1, or an empty vector. Primary antibodies were detected using FITC-conjugated secondary antibodies. The samples were analyzed on a FACSCalibur using CellQuest Pro Version 6 software (BD Biosciences) and Flowing-Software Version 2.4.7 (Perttu Terho, Center for Biotechnology; www.flowingsoftware.com).
For antibodies and staining procedures, see supplemental Methods.
Immunoprecipitations and immunoblottings
For Western blot analyses, lymphocyte-depleted tonsil lysate and HEK-PV-1 transfectants were run on SDS-PAGE under nonreducing conditions, transferred to nitrocellulose membranes, and incubated with primary antibodies followed by HRP-conjugated anti–mouse IgG (Dako Denmark) or anti–goat IgG (Santa Cruz Biotechnology).
Coimmunoprecipitations were performed as described10 using fresh lymphocyte-depleted tonsil lysate or PV-1– and NRP-1–cotransfected HEK EBNA cell lysate. Magnetic beads (Dynal) were coated with 174/2 (anti–PV-1 antibody), polyclonal α-NRP-1, AK-1 (negative control), or normal goat serum (Vector Laboratories). Precipitated proteins were analyzed as described for Western blot analysis, but run under reducing conditions on SDS-PAGE.
Results and discussion
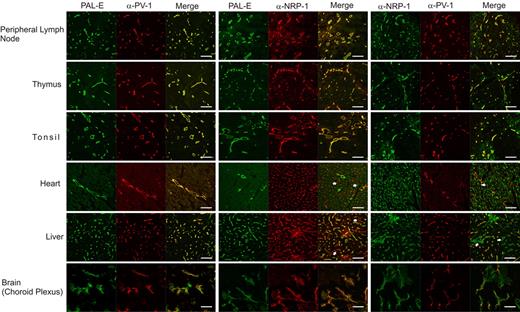
First, we used immunofluorescence to study whether PAL-E and antibodies against PV-1 and NRP-1 would reveal differences in the expression patterns of their respective target molecules. For that purpose, we stained human peripheral lymph node, liver, thymus, brain (choroid plexus), tonsil, and heart tissues. As PV-1 is absent from normal brain endothelium and is up-regulated only after blood-brain barrier disruption,11 we used choroid plexus, a part of the brain characterized by its fenestrated endothelium,12 expressing PV-1.
These stainings showed that PV-1 and the epitope of PAL-E colocalize perfectly in every analyzed tissue. However, double stainings of PAL-E and NRP-1 or PV-1 and NRP-1 revealed significant differences in the heart and liver (Figure 1; supplemental Videos). To confirm the nature of the stained structures, we performed double stainings of PV-1, NRP-1, and PAL-E with an antibody against the prototype vessel marker CD31/PECAM-1. Indeed, all structures stained by anti–PV-1 antibodies and PAL-E were also positive for CD31 (supplemental Figure 1). Only the antibody against NRP-1 stained some nonvascular structures in the liver.
PAL-E antigen colocalizes with PV-1 and NRP-1. The 2-color immunofluorescent stainings of the indicated human tissues using anti–PV-1, anti–NRP-1, and PAL-E antibodies were performed as described in “Immunostainings” and, in more detail, in supplemental Methods. White arrows indicate the structures, which are positively stained by anti–NRP-1 antibodies but negative for both anti–PV-1 and PAL-E. Original magnifications ×200. Scale bars represent 100 μm.
PAL-E antigen colocalizes with PV-1 and NRP-1. The 2-color immunofluorescent stainings of the indicated human tissues using anti–PV-1, anti–NRP-1, and PAL-E antibodies were performed as described in “Immunostainings” and, in more detail, in supplemental Methods. White arrows indicate the structures, which are positively stained by anti–NRP-1 antibodies but negative for both anti–PV-1 and PAL-E. Original magnifications ×200. Scale bars represent 100 μm.
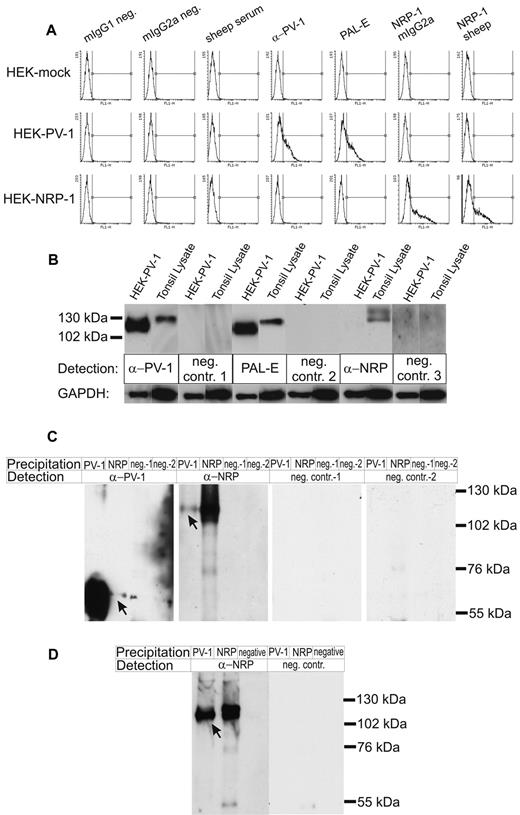
Next, we used HEK EBNA cells transiently transfected with PV-1, NRP-1, or mock plasmids to determine the binding of the antibody PAL-E. As expected, the anti–PV-1 antibody positively stained the PV-1 transfectants, whereas 2 different anti–NRP-1 antibodies bound specifically to the NRP-1–transfected cells. The PAL-E antibody, on the other hand, positively stained PV-1 transfectants, whereas NRP-1–expressing cells remained negative (Figure 2A).
PAL-E recognizes PV-1, which can form complexes with NRP-1. (A) HEK-EBNA cells mock transfected (HEK-mock), transfected with PV-1 (HEK-PV-1) or NRP-1 (HEK-NRP-1), were stained with the indicated antibodies and analyzed by flow cytometry. In the histograms, the x-axis represents the fluorescence intensity; and the y-axis, the number of cells. (B) Immunoblots of HEK-PV-1 transfectants and tonsil lysate. The indicated antibodies followed by HRP-conjugated anti–mouse IgG or anti–sheep IgG were used for chemiluminescence detection (neg. contr. 1 and neg. contr. 2 indicate controls for anti–PV-1 and PAL-E, respectively; and neg. contr. 3, control for anti–NRP-1). Gels were run under nonreducing conditions, allowing the maintenance of PV-1 homodimers, resulting in PV-1 bands of approximately 110 to 130 kDa and NRP-1 bands of approximately 130 to 140 kDa. (C) Coimmunoprecipitations with anti–PV-1 and anti–NRP-1 antibodies. The precipitating and detecting antibodies are indicated (neg.-1 or neg. contr.-1 indicate the negative control antibody for anti–PV-1; and neg.-2 or neg. contr.-2, the negative control antibody for anti–NRP-1, respectively). Gels were run under reducing conditions, thus showing monomeric PV-1 bands of approximately 55 to 65 kDa and NRP-1 bands of approximately 130 kDa. Arrows point to the coprecipitated proteins in lane 2 and lane 5. (D) HEK-EBNA cells transiently cotransfected with PV-1 and NRP-1 were used to confirm the results of the coimmunoprecipitation from fresh tonsil lysate. Gels were run under reducing conditions, thus showing monomeric PV-1 bands of approximately 55 to 65 kDa and NRP-1 bands of approximately 130 kDa. The arrow indicates the coprecipitated protein in lane 1. Bands in the range of 50 kDa stem from the heavy chain of the immunoglobulins. The experiments were performed 3 times (A) and twice (B-D) with comparable results.
PAL-E recognizes PV-1, which can form complexes with NRP-1. (A) HEK-EBNA cells mock transfected (HEK-mock), transfected with PV-1 (HEK-PV-1) or NRP-1 (HEK-NRP-1), were stained with the indicated antibodies and analyzed by flow cytometry. In the histograms, the x-axis represents the fluorescence intensity; and the y-axis, the number of cells. (B) Immunoblots of HEK-PV-1 transfectants and tonsil lysate. The indicated antibodies followed by HRP-conjugated anti–mouse IgG or anti–sheep IgG were used for chemiluminescence detection (neg. contr. 1 and neg. contr. 2 indicate controls for anti–PV-1 and PAL-E, respectively; and neg. contr. 3, control for anti–NRP-1). Gels were run under nonreducing conditions, allowing the maintenance of PV-1 homodimers, resulting in PV-1 bands of approximately 110 to 130 kDa and NRP-1 bands of approximately 130 to 140 kDa. (C) Coimmunoprecipitations with anti–PV-1 and anti–NRP-1 antibodies. The precipitating and detecting antibodies are indicated (neg.-1 or neg. contr.-1 indicate the negative control antibody for anti–PV-1; and neg.-2 or neg. contr.-2, the negative control antibody for anti–NRP-1, respectively). Gels were run under reducing conditions, thus showing monomeric PV-1 bands of approximately 55 to 65 kDa and NRP-1 bands of approximately 130 kDa. Arrows point to the coprecipitated proteins in lane 2 and lane 5. (D) HEK-EBNA cells transiently cotransfected with PV-1 and NRP-1 were used to confirm the results of the coimmunoprecipitation from fresh tonsil lysate. Gels were run under reducing conditions, thus showing monomeric PV-1 bands of approximately 55 to 65 kDa and NRP-1 bands of approximately 130 kDa. The arrow indicates the coprecipitated protein in lane 1. Bands in the range of 50 kDa stem from the heavy chain of the immunoglobulins. The experiments were performed 3 times (A) and twice (B-D) with comparable results.
In immunoblotting (Figure 2B), PAL-E and an anti–PV-1 antibody both recognized bands of the same size (∼ 110-130 kDa) when lysates from HEK–PV-1 transfectants and tonsil were analyzed under nonreducing conditions. The anti–NRP-1 antibody recognized a specific protein band only in tonsil lysate. The absence of a signal in the HEK–PV-1 transfectants shows that the anti–NRP-1 antibody does not recognize PV-1.
To study whether PV-1 associates with NRP-1, we performed coimmunoprecipitations from fresh tonsil lysate. We found that NRP-1 can be precipitated using anti–PV-1 antibodies and immunoprecipitation with anti–NRP-1 antibodies specifically pulls down some PV-1 (Figure 2C). As the amount of PV-1 precipitated with α-NRP-1 antibodies was very low, we confirmed the physical association of PV-1 and NRP-1 using PV-1 precipitation followed by detection of NRP-1 in transiently transfected HEK-EBNA cells (Figure 2D).
Although Jaalouk et al reported that PAL-E recognizes NRP-1,4 the expression of NRP-1 in multiple nonvascular cells, including neuronal structures, thymic epithelial cells, T cells, and dendritic cells on one hand13,14 and the widespread use of PAL-E as a marker for vascular endothelium more than 2 decades on the other hand, are in disagreement with this interpretation. They further mapped the PAL-E binding to the SQYSTNW motif (spanning residues 295-301) in NRP-1, which overlaps with the VEGF ligand binding site predicted earlier.15 PV-1 has no similar sequence motif. In our modelings of the 3-dimensional structure, PV-1 was predicted to be totally α-helical and PV-1 did not show any structural similarity to NRP-1. Thus, a possible structural similarity between PV-1 and NRP-1 cannot explain the false identification of NRP-1 as a PAL-E antigen.
Furthermore, the argument of Jaalouk et al,4 claiming that anti–PV-1 and PAL-E antibodies cannot recognize the same molecule as they fail to inhibit each other in competitive staining experiments, is invalidated by the fact that all glycoproteins carry multiple antigenic epitopes.
The function and expression of NRP-1 and VEGFR-2 in endothelial cells are closely associated.8,16,17 In addition, a recent publication demonstrated that NRP-1 ligands, such as VEGF-A165, contain a sequence interacting with NRP-1 and causing vascular leakage and cellular internalization.18 The authors also suggested that these functions might involve vesiculo-vacuolar organelles (VVOs) because VEGFR-2 can be detected on the luminal and abluminal surfaces as well as on the membranes of VVOs. Furthermore, VEGFR-2 was found on caveolae and the stomatal diaphragms of some VVOs,19 which are formed by PV-1.20 VVOs are located at sites of neutrophil transcellular diapedesis and thought to facilitate this process.21 In addition, VEGF-A165 has been shown to regulate PV-1 expression via VEGFR-2.22,23 Finally, a recent report demonstrated a function for NRP-1 during cancer cell migration.24 Based on our findings and these reports, it is probable that NRP-1 may functionally be linked to PV-1.
In conclusion, we show that the prototype endothelial marker PAL-E recognizes PV-1. However, PV-1 and NRP-1 colocalize to a very high degree and can physically interact. Hence, we connect, for the first time, these 2 proteins: one involved in leukocyte trafficking and the second in angiogenesis and cancer cell migration. This opens interesting directions for further studies in vascular biology and angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.K. designed and performed experiments, analyzed data, and wrote the manuscript; D.T. provided vital reagents; K.E. designed experiments and analyzed data; M.S. designed experiments, analyzed data, and edited the manuscript; K.A. provided vital reagents and expertise and edited the manuscript; T.S. made the structural analyses; and S.J. designed the study and experiments, analyzed data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sirpa Jalkanen, MediCity Research Laboratory, University of Turku, Tykistökatu 6 A, FIN-20520 Turku, Finland; e-mail: sirpa.jalkanen@utu.fi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal