Abstract
Acquired hemophilia A is a rare bleeding disorder caused by autoantibodies to coagulation FVIII. Bleeding episodes at presentation are spontaneous and severe in most cases. Optimal hemostatic therapy is controversial, and available data are from observational and retrospective studies only. The EACH2 registry, a multicenter, pan-European, Web-based database, reports current patient management. The aim was to assess the control of first bleeding episodes treated with a bypassing agent (rFVIIa or aPCC), FVIII, or DDAVP among 501 registered patients. Of 482 patients with one or more bleeding episodes, 144 (30%) received no treatment for bleeding; 31 were treated with symptomatic therapy only. Among 307 patients treated with a first-line hemostatic agent, 174 (56.7%) received rFVIIa, 63 (20.5%) aPCC, 56 (18.2%) FVIII, and 14 (4.6%) DDAVP. Bleeding was controlled in 269 of 338 (79.6%) patients treated with a first-line hemostatic agent or ancillary therapy alone. Propensity score matching was applied to allow unbiased comparison between treatment groups. Bleeding control was significantly higher in patients treated with bypassing agents versus FVIII/DDAVP (93.3% vs 68.3%; P = .003). Bleeding control was similar between rFVIIa and aPCC (93.0%; P = 1). Thrombotic events were reported in 3.6% of treated patients with a similar incidence between rFVIIa (2.9%) and aPCC (4.8%).
Introduction
Acquired hemophilia A (AHA) is a hemorrhagic syndrome characterized by a deficiency of coagulation factor VIII (FVIII) secondary to autoantibodies targeting specific epitopes that cause neutralization and/or accelerated clearance of FVIII from the plasma.1 The reported incidence of AHA varies between 0.1 and 1.5 cases per million2 population, although a study in a defined population suggests an incidence of 1.5 per million.3 AHA is commonly associated with a variety of clinical conditions, including autoimmune disorders, solid tumors, lymphoproliferative diseases, and pregnancy; however, no underlying condition is identified in approximately 50% of the cases.1-3
Hemorrhages occur in AHA patients without a family or personal history of bleeding and usually exhibit a sudden onset. Bleeding episodes are spontaneous in the majority of cases, although approximately 25% of cases occur after trauma or invasive procedures.4 Retroperitoneal and large intramuscular hematomas may compress nervous and vascular structures, leading to compartmental syndromes. Bleeding at presentation is usually severe (> 67% of the cases) but may be mild, and approximately 25% of patients do not require hemostatic treatment.2,5
Observed clinical bleeding does not correlate with FVIII level or inhibitor titer and differs from hereditary hemophilia: skin, mucous membranes, muscles, and soft tissue bleeds are more common, whereas hemarthroses are unusual. The mortality rate resulting from bleeding or otherwise related to AHA is high and reported to be between 7.9% and 22%.1-3,5,6
The hemostatic agents most frequently used to control bleeding in AHA are bypassing agents, recombinant activated FVII (rFVIIa) or activated prothrombin complex concentrate (aPCC), or FVIII replacement therapy with concentrates or induction of FVIII release using 1-desamino-8-D-arginine-vasopressin (DDAVP).7 The optimal therapy is controversial, and available data are derived from observational and retrospective studies, including a limited number of patients with different primary clinical conditions.7-12
To address this problem, the European Acquired Hemophilia (EACH2) registry was established in 2003 to collect information on the current management of patients with AHA. No treatment protocol was provided, and patients were managed according to local clinical practice. This primary analysis focuses on the hemostatic treatment of bleeding episodes with rFVIIa, aPCC, FVIII, or DDAVP.
Methods
A total of 117 centers in 13 European countries participated in the Web-based registry. The contributing centers are listed in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The registry was hosted by Parexel International GmbH, supported by an unrestricted grant from Novo Nordisk Region Europe A/S, and reviewed by institutional review boards at each center. Informed consent was obtained from all patients or their next of kin in accordance with the Declaration of Helsinki. The study was longitudinal, prospective, and observational and registered consecutive patients. Patient characteristics at diagnosis were recorded and have been previously detailed.13 The characteristics and outcome of patients who received immunosuppressive treatment have also been reported.14 Data for each bleeding episode were entered separately. The following characteristics of each new bleed were reported: site (CNS, musculoskeletal, retroperitoneal, gastrointestinal, urogenital, skin, none), cause (spontaneous, traumatic, surgical, peripartum or postpartum), severity (severe defined as life-, limb-, or organ-threatening, CNS, hemoglobin [Hb] < 8 g/dL or a drop of > 2 g/dL; RBC transfusion requirement of > 2 units in 24 hours). All other bleeding episodes were defined as nonsevere. The Hb, activated partial thromboplastin time, FVIII level, and inhibitor titer were recorded at presentation and/or at the time of the bleed.
The hemostatic therapy used was described by agent and dosage regimen (initial dose per bolus, initial interval of successive doses, number of doses and total dose, total days of treatment). For patients treated with aPCC, FVIII, or DDAVP, anamnestic response was reported as yes or no. The following ancillary therapies were recorded: antifibrinolytic agents, RBC transfusion, topical therapy, immunoadsorption, plasmapheresis, and high-dose immunoglobulins. The response to therapy was judged clinically by the local investigator and was recorded as bleeding resolved with date or bleeding not resolved. Secondary treatment was initiated because of lack of efficacy or other reasons and was reported using the same data points. Adverse events, including thrombosis, that in the opinion of the investigator were related to hemostatic therapy, were reported. All bleeding episodes were recorded in the registry, but only first bleeding episodes are analyzed here.
Baseline patient characteristics are reported as frequency (percentage) or median and interquartile range (IQR) for categorical and continuous variables, respectively. The primary endpoint was control of the first bleeding episode treated with first-line hemostatic therapy. Only first bleeds and first-line treatment are reported to avoid any potential indication bias. Reporting of all episodes experienced and therapy lines administered could impair treatment comparison because treatment assignment is highly conditional on previous treatment and nonresponsiveness (ie, correlated end points and time-dependent confounding). The first-line hemostatic therapy options analyzed were rFVIIa, aPCC, FVIII, and DDAVP. Initial treatment regimens and response are reported descriptively, however, because the baseline characteristics of patients and bleeding episodes vary widely, the results cannot be directly compared. To make comparisons between treatment strategies, baseline characteristics needed to be matched.
Two separate treatment comparisons were assessed. First, bypassing agents (rFVIIa or aPCC) versus FVIII or DDAVP were compared; then a specific comparison between rFVIIa and aPCC was performed. Prematching comparisons between groups were performed using a Pearson χ2 test or Mann-Whitney U test. To allow an unbiased comparison between the treatment groups, propensity score (PS) methodology15,16 was used.
For each analysis, a separate logistic regression model was first applied to predict the probability (PS) of receiving either treatment (bypassing agents vs FVIII replacement or release in the first analysis; rFVIIa vs aPCC in the second). Both models included the same set of variables: age at diagnosis, sex, factor VIII level and inhibitor titer at time of the bleeding episode, Hb value at diagnosis, bleeding site (CNS, deep, hemarthroses, mucosa, skin, multiple sites), bleeding severity (severe vs nonsevere), delay of therapy, and cause of bleeding (spontaneous vs traumatic).
Consequently, PSs derived from the logistic models were used within a 5 → 1 greedy 1:1 matching algorithm15 to obtain the matched samples. The greedy matching algorithm generated matched pairs of patients identical in their PS value, decreasing from 5 decimal places to 1. Adequacy of covariate balance in the matched samples was eventually assessed with McNemar χ2 or a Wilcoxon signed-rank test. Rates of bleeding episodes not resolved with first-line therapy were reported for unmatched and matched samples. PS-matched logistic models were expressed as odds ratios (OR) along with 95% confidence intervals (CI) and were derived from a generalized estimating equations logistic regression to account for the matched design. Where appropriate to assess robustness of findings a PS quintiles-adjusted model was also used.16
Last, because PS methodology only addresses imbalances resulting from measured covariates, we also performed a sensitivity analysis17 on significant findings to account for potential residual confounding because of an unknown, unmeasured baseline covariate. P values less than .05 were considered significant. All the analyses were performed using SAS Version 9.1 (SAS Institute).
Results
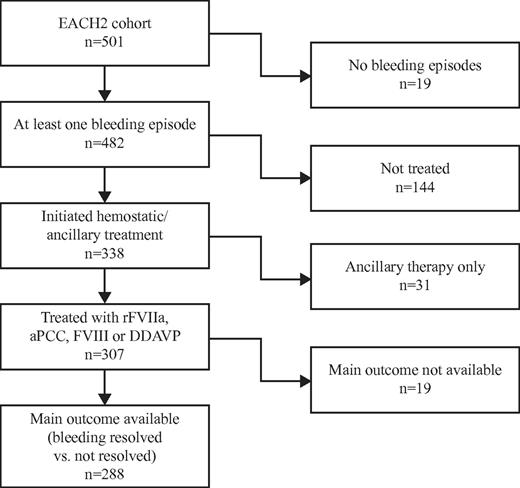
Between January 2003 and December 2008, 501 patients from 117 centers in 13 European countries (Austria, Finland, France, Germany, Greece, Hungary, Italy, The Netherlands, Portugal, Spain, Sweden, Switzerland, and United Kingdom) were entered into the database. The demographics and characteristics at presentation of the entire cohort have been reported previously.13 A total of 482 patients experienced at least 1 bleeding episode and 19 (3.8%) reported no bleeding. The baseline characteristics of the patients who bled and those who did not bleed were similar with respect to age, sex, weight, Hb, FVIII level, inhibitor titer, and associated underlying conditions. Among the 482 patients who bled 230 (47.7%) were female and 252 (52.3%) were male. The median (IQR) values for patients who bled were as follows: age, 74.0 years (61.0-80.0 years); weight, 69.4 kg (60.0-78.0 kg); FVIII activity, 2.0 IU/dL (1.0-6.0 IU/dL); Hb, 9.0 g/dL (7.5-11.3 g/dL); and FVIII inhibitor titer, 14.0 (5.0-42.0) Bethesda units (BU)/mL. The underlying medical condition was reported as an autoimmune disorder in 65 (14.1%), malignancy in 53 (11.5%), pregnancy in 41 (8.9%), drug-induced in 15 (3.3%), and transfusion, dermatologic, infection, monoclonal gammopathy of undetermined significance, or other in 36 (7.8%). No associated medical condition was identified (idiopathic) in 250 (54.4%), and no information was reported for 22 patients (4.6%). The disposition of patients in the cohort with respect to bleeding and hemostatic treatment is shown in Figure 1.
Of the 482 patients with at least one bleeding episode, 144 (30%) received no hemostatic treatment. Treated and untreated patients differed only in Hb level (8.6 vs 10.9 g/dL), site, and severity of bleeding (all P < .0001). Age, sex, FVIII level, inhibitor titer, cause of bleeding, and underlying conditions were similar. The nontreated group included 94 nonsevere bleeding episodes and 47 severe bleeds. The reason for not treating patients was not recorded in the registry. Among the patients who experienced a bleeding episode but were not treated, the Hb level (11.0 vs 9.4 g/dL; P = .0004), cause (spontaneous vs nonspontaneous; 86.2% vs 72.3%; P = .0459), and site of bleeding (deep, 14.9% vs 46.8%; skin, 62.7% vs 17.0%; mucosal, 21.3% vs 34.0%; P < .0001) differed between nonsevere and severe bleeding episodes. A total of 338 patients received treatment for a first bleed, but 31 (9.2%) were treated with ancillary therapy only (1 immunoadsorption, 13 antifibrinolytic, 17 RBC, 3 topical therapy, 4 high-dose intravenous Ig, and 12 other). A total of 307 patients received a first-line hemostatic agent: 174 were treated with rFVIIa (56.7%), 63 with aPCC (20.5%), 56 with FVIII (18.2%), and 14 with DDAVP (4.6%). Among patients treated with ancillary therapy only compared with those who received a hemostatic agent, a lower FVIII level at the time of the bleed was noted for the latter (5.9 vs 4.1 IU/dL; P = .0334). Fewer nonspontaneous bleeds (45.2% vs 20.9%; P = .0023) were reported among those receiving ancillary therapy only. The site of bleeding (deep, 48.4% vs 60.9%; mucosal, 41.9% vs 18.6%; P = .0661) and severity of bleeding (25.9% vs 13.4%; P = .0618) showed a trend toward significance. Bleeding resolved in 22 of 31 patients treated with ancillary therapy only; 6 of 9 patients who did not respond were switched to a bypassing agent, and 3 of these episodes resolved. Among the 22 patients with resolved bleeding episodes after ancillary therapy alone, 10 patients received antifibrinolytic therapy, 9 RBC, 3 high-dose intravenous Ig, 1 immunoadsorption, and 2 topical therapy. Overall, ancillary therapy, including that administered to the 307 patients also receiving hemostatic therapy, was composed of 20 (5.9%) patients receiving immunoadsorption, 2 (0.6%) plasmapheresis, 64 (18.9%) antifibrinolytic agents, 184 (54.4%) RBC, 10 (3.0%) topical therapy, 38 (11.2%) high-dose intravenous Ig, and 53 (15.7%) other therapies. The overall median number of RBC units transfused was 4 (IQR, 2-8 units), with no differences observed between site, severity, cause, and bleed resolution.
Bleeding was controlled in 269 patients (79.6%), including those treated with ancillary therapy only. The only parameter that significantly differed between patients who responded to treatment and those who did not was a delay in time to treatment (median, 1.00 vs 4.00 days; P = .0155). The FVIII level in responders and nonresponders at the time of the first bleeding episode did not differ, nor did inhibitor titer (10.0 vs 13.0; P = .9294), bleeding cause (traumatic 13.2% vs spontaneous 15.7%; P = .5888), bleeding site (P = .2939), or severity (15.9% vs 10.4%; P = .3259).
Among the 69 patients who received second-line therapy, 50 (14.8%) were treated because of lack of efficacy of first-line treatment and 19 (5.6%) for unspecified reasons, 24 (36.9%) were treated with rFVIIa, 15 (23.1%) with aPCC, 23 (35.4%), with FVIII 1 (1.5%) with DDAVP, 2 (3.1%) received ancillary therapy only, and the type of agent was not reported for 4 patients. Information on the response to second-line treatment was available for 68 patients. Bleeding resolved in 54 patients (79.4%). Eleven (16.2%) did not resolve and initiated third-line therapy for lack of efficacy; 3 patients (4.4%) initiated third-line therapy for unspecified reasons other than lack of efficacy. Information on bleeding recurrence was available for 65 of 69 second-line therapy patients: 18 patients (27.7%) had a recurrence. Of the 269 patients in whom bleeding resolved with first-line therapy, 68 patients had a recurrence (99 episodes) with an overall recurrence rate of 25.3% (median inhibitor titer, 8.6 BU/mL; 2.0-37.0 BU/mL). The median time to recurrence was 14 days (IQR, 3-45 days).
The unmatched and matched baseline covariates comparing bypassing agents (n = 219) and FVIII replacement and DDAVP (n = 69) according to treatment of the 288 patients for whom the primary outcome data were available are reported in Tables 1 and 2. FVIII level, inhibitor titer, bleeding sites, and severity of bleeding were significantly different in the 2 unmatched groups; sex showed a trend toward significance. A total of 219 patients were treated with bypassing agents (159 with rFVIIa, 60 with aPCC) and 69 with FVIII (n = 55) or DDAVP (n = 14). Bleeding was controlled in 201 patients treated with bypassing agents (91.8%) and 48 patients (69.6%) treated with replacement therapy (P < .003). Among the patients treated with replacement therapy, bleeding was resolved in 39 patients (70.1%) treated with FVIII and 9 (64.3%) of those treated with DDAVP. PS 1:1 matching allowed an unbiased comparison between the 2 groups by matching 60 of 69 available pairs. The baseline covariates after PS matching were balanced. In the matched samples, 56 of 60 patients (93.3%) in the group treated with bypassing agents and 41 of 60 (68.3%) in the group treated with FVIII or DDAVP, respectively, experienced bleeding episodes that were controlled by first-line therapy (OR = 0.15; 95% CI, 0.04-0.53; P = .003). The hemostatic effect of each of the agents is shown in Table 3.
Unmatched sample baseline characteristics according to treatment of the first bleeding episode (bypassing agent vs FVIII or DDAVP and rFVIIa vs aPCC)
| Variable . | Bypassing agent, median (IQR) . | FVIII or DDAVP, median (IQR) . | P* . | rFVIIa, median (IQR) . | aPCC, median (IQR) . | P† . |
|---|---|---|---|---|---|---|
| Patients, n | 219 | 69 | 159 | 60 | ||
| Age, y | 73.0 (15.0-92.0) | 73.0 (13.0-104.0) | .94 | 73.0 (15.0-91.0) | 76.5 (24.0-92.0) | .02 |
| Sex, n (%) | .07 | .06 | ||||
| Female | 109 (49.7) | 26 (37.7) | 73 (45.9) | 36 (60.0) | ||
| Male | 110 (50.8) | 43 (62.3) | 86 (54.1) | 24 (40.0) | ||
| Weight, kg | 69.0 (40.0-130.0) | 69.0 (40.0-113.0) | .92 | 69.0 (40.0-130.0) | 69.2 (44.0-107.0) | .70 |
| FVIII level, IU/dL | 1.0 (0.0-40.0) | 3.0 (0.0-34.0) | .03 | 2.0 (0.0-32.0) | 1.0 (0.0-40.0) | .13 |
| Hb, g/dL | 8.6 (3.0-15.2) | 8.8 (3.3-14.4) | .57 | 8.6 (3.0-15.2) | 8.4 (4.6-14.8) | .90 |
| Inhibitor titer, BU/mL | 15.4 (0.1-2765.0) | 8.0 (0.3-200.0) | .0003 | 15.0 (1.0-2765.0) | 17.0 (0.1-1700.0) | .99 |
| Therapy delay, days | 0.01 (0.0-0.5) | 0.01 (0.00-0.11) | .34 | 0.01 (0.00-0.27) | 0.01 (0.00-0.54) | .76 |
| Ancillary antifibrinolytic therapy, n (%) | 30 (13.7) | 20 (29.0)‡ | .0035 | 27 (17.0) | 3 (5.0) | .0215 |
| Cause of bleeding, n (%) | .715 | .08 | ||||
| Unknown | 1 | 0 | 1 | 0 | ||
| Traumatic | 46 (21.1) | 16 (23.2) | 38 (24.1) | 8 (13.3) | ||
| Spontaneous | 172 (78.9) | 53 (76.8) | 120 (75.9) | 52 (86.7) | ||
| Bleeding site, n (%) | .04 | .12 | ||||
| CNS | 5 (2.3) | 0 (0.0) | 5 (3.1) | 0 (0.0) | ||
| Deep muscle | 139 (63.4) | 32 (46.4) | 94 (59.1) | 45 (75.0) | ||
| Hemarthrosis | 6 (2.7) | 3 (4.3) | 5 (3.1) | 1 (1.7) | ||
| Mucosa | 34 (15.6) | 21 (30.5) | 30 (18.8) | 4 (6.6) | ||
| Skin | 34 (15.6) | 13 (18.8) | 24 (15.2) | 10 (16.7) | ||
| Multiple sites | 1 (0.4) | 0 (0.0) | 1 (0.7) | 0 (0.0) | ||
| Severity of bleeding, n (%) | .031 | .31 | ||||
| Unknown | 1 | 0 | 1 | 0 | ||
| Severe | 193 (88.5) | 54 (78.2) | 142 (89.8) | 51 (85.0) | ||
| Nonsevere | 25 (11.5) | 15 (21.8) | 16 (10.1) | 9 (15.0) | ||
| Variable . | Bypassing agent, median (IQR) . | FVIII or DDAVP, median (IQR) . | P* . | rFVIIa, median (IQR) . | aPCC, median (IQR) . | P† . |
|---|---|---|---|---|---|---|
| Patients, n | 219 | 69 | 159 | 60 | ||
| Age, y | 73.0 (15.0-92.0) | 73.0 (13.0-104.0) | .94 | 73.0 (15.0-91.0) | 76.5 (24.0-92.0) | .02 |
| Sex, n (%) | .07 | .06 | ||||
| Female | 109 (49.7) | 26 (37.7) | 73 (45.9) | 36 (60.0) | ||
| Male | 110 (50.8) | 43 (62.3) | 86 (54.1) | 24 (40.0) | ||
| Weight, kg | 69.0 (40.0-130.0) | 69.0 (40.0-113.0) | .92 | 69.0 (40.0-130.0) | 69.2 (44.0-107.0) | .70 |
| FVIII level, IU/dL | 1.0 (0.0-40.0) | 3.0 (0.0-34.0) | .03 | 2.0 (0.0-32.0) | 1.0 (0.0-40.0) | .13 |
| Hb, g/dL | 8.6 (3.0-15.2) | 8.8 (3.3-14.4) | .57 | 8.6 (3.0-15.2) | 8.4 (4.6-14.8) | .90 |
| Inhibitor titer, BU/mL | 15.4 (0.1-2765.0) | 8.0 (0.3-200.0) | .0003 | 15.0 (1.0-2765.0) | 17.0 (0.1-1700.0) | .99 |
| Therapy delay, days | 0.01 (0.0-0.5) | 0.01 (0.00-0.11) | .34 | 0.01 (0.00-0.27) | 0.01 (0.00-0.54) | .76 |
| Ancillary antifibrinolytic therapy, n (%) | 30 (13.7) | 20 (29.0)‡ | .0035 | 27 (17.0) | 3 (5.0) | .0215 |
| Cause of bleeding, n (%) | .715 | .08 | ||||
| Unknown | 1 | 0 | 1 | 0 | ||
| Traumatic | 46 (21.1) | 16 (23.2) | 38 (24.1) | 8 (13.3) | ||
| Spontaneous | 172 (78.9) | 53 (76.8) | 120 (75.9) | 52 (86.7) | ||
| Bleeding site, n (%) | .04 | .12 | ||||
| CNS | 5 (2.3) | 0 (0.0) | 5 (3.1) | 0 (0.0) | ||
| Deep muscle | 139 (63.4) | 32 (46.4) | 94 (59.1) | 45 (75.0) | ||
| Hemarthrosis | 6 (2.7) | 3 (4.3) | 5 (3.1) | 1 (1.7) | ||
| Mucosa | 34 (15.6) | 21 (30.5) | 30 (18.8) | 4 (6.6) | ||
| Skin | 34 (15.6) | 13 (18.8) | 24 (15.2) | 10 (16.7) | ||
| Multiple sites | 1 (0.4) | 0 (0.0) | 1 (0.7) | 0 (0.0) | ||
| Severity of bleeding, n (%) | .031 | .31 | ||||
| Unknown | 1 | 0 | 1 | 0 | ||
| Severe | 193 (88.5) | 54 (78.2) | 142 (89.8) | 51 (85.0) | ||
| Nonsevere | 25 (11.5) | 15 (21.8) | 16 (10.1) | 9 (15.0) | ||
P values refer to Pearson χ2 or Mann-Whitney U test for the comparison between bypassing agent and FVIII or DDAVP.
P values refer to Pearson χ2 or Mann-Whitney U test for the comparison between rFVIIa and aPCC.
A total of 13 of 55 patients (23.6%) treated with FVIII received ancillary antifibrinolytic treatment; 7 of 14 (50%) of patients treated with DDAVP also received antifibrinolytics.
Matched sample baseline characteristics according to treatment of the first bleeding episode (bypassing agent vs FVIII or DDAVP and rFVIIa vs aPCC)
| Variable . | Bypassing agent, median (IQR) . | FVIII or DDAVP, median (IQR) . | P* . | rFVIIa,† median (IQR) . | aPCC, median (IQR) . | P* . |
|---|---|---|---|---|---|---|
| Patients, n | 60 | 60 | 57 | 57 | ||
| Age, y | 74.0 (24.0-91.0) | 72.5 (13.0-104.0) | .95 | 72.00 (39.00-91.00) | 77.00 (24.00-92.00) | .41 |
| Sex, n (%) | .69 | .41 | ||||
| Female | 25 (41.7) | 23 (38.3) | 37 (64.91) | 33 (57.89) | ||
| Male | 35 (58.3) | 37 (61.7) | 20 (35.09) | 24 (42.11) | ||
| Weight, kg | 70.0 (40.0-107.0) | 68.0 (40.0-113.0) | .49 | 70.00 (40.00-120.00) | 70.00 (44.00-107.00) | .66 |
| FVIII level, IU/dL | 2.0 (0.0-40.0) | 3.0 (0.0-34.0) | .61 | 1.25 (0.00-32.00) | 1.00 (0.00-40.00) | .41 |
| Hb, g/dL | 8.4 (3.0-14.2) | 8.8 (3.3-14.4) | .41 | 8.50 (3.00-14.00) | 8.40 (4.60-14.80) | .84 |
| Inhibitor titer, BU/mL | 9.3 (1.0-2765.0) | 8.0 (0.3-200.0) | .52 | 16.00 (1.00-2765.00) | 17.00 (0.10-1700.00) | .52 |
| Therapy delay, d | 0.01 (0.0-0.13) | 0.01 (0.0-0.11) | .46 | 0.01 (0.00-0.09) | 0.01 (0.00-0.54) | .64 |
| Cause of bleeding, n (%) | .51 | .62 | ||||
| Traumatic | 16 (26.7) | 13 (21.7) | 10 (17.54) | 8 (14.04) | ||
| Spontaneous | 44 (73.3) | 47 (78.3) | 47 (82.46) | 49 (85.96) | ||
| Bleeding site, n (%) | .99 | .55 | ||||
| Deep | 30 (50.0) | 30 (50.0) | 44 (77.19) | 44 (77.19) | ||
| Hemarthrosis | 3 (5.0) | 2 (3.3) | 0 (0.00) | 1 (1.75) | ||
| Mucosa | 15 (25.0) | 16 (26.7) | 5 (8.77) | 4 (7.02) | ||
| Skin | 12 (20.0) | 12 (20.0) | 8 (14.04) | 8 (14.04) | ||
| Severity of bleeding, n (%) | .63 | .56 | ||||
| Severe | 47 (78.3) | 49 (81.7) | 49 (85.96) | 51 (89.47) | ||
| Nonsevere | 13 (21.6) | 11 (18.3) | 8 (14.04) | 6 (10.53) | ||
| Variable . | Bypassing agent, median (IQR) . | FVIII or DDAVP, median (IQR) . | P* . | rFVIIa,† median (IQR) . | aPCC, median (IQR) . | P* . |
|---|---|---|---|---|---|---|
| Patients, n | 60 | 60 | 57 | 57 | ||
| Age, y | 74.0 (24.0-91.0) | 72.5 (13.0-104.0) | .95 | 72.00 (39.00-91.00) | 77.00 (24.00-92.00) | .41 |
| Sex, n (%) | .69 | .41 | ||||
| Female | 25 (41.7) | 23 (38.3) | 37 (64.91) | 33 (57.89) | ||
| Male | 35 (58.3) | 37 (61.7) | 20 (35.09) | 24 (42.11) | ||
| Weight, kg | 70.0 (40.0-107.0) | 68.0 (40.0-113.0) | .49 | 70.00 (40.00-120.00) | 70.00 (44.00-107.00) | .66 |
| FVIII level, IU/dL | 2.0 (0.0-40.0) | 3.0 (0.0-34.0) | .61 | 1.25 (0.00-32.00) | 1.00 (0.00-40.00) | .41 |
| Hb, g/dL | 8.4 (3.0-14.2) | 8.8 (3.3-14.4) | .41 | 8.50 (3.00-14.00) | 8.40 (4.60-14.80) | .84 |
| Inhibitor titer, BU/mL | 9.3 (1.0-2765.0) | 8.0 (0.3-200.0) | .52 | 16.00 (1.00-2765.00) | 17.00 (0.10-1700.00) | .52 |
| Therapy delay, d | 0.01 (0.0-0.13) | 0.01 (0.0-0.11) | .46 | 0.01 (0.00-0.09) | 0.01 (0.00-0.54) | .64 |
| Cause of bleeding, n (%) | .51 | .62 | ||||
| Traumatic | 16 (26.7) | 13 (21.7) | 10 (17.54) | 8 (14.04) | ||
| Spontaneous | 44 (73.3) | 47 (78.3) | 47 (82.46) | 49 (85.96) | ||
| Bleeding site, n (%) | .99 | .55 | ||||
| Deep | 30 (50.0) | 30 (50.0) | 44 (77.19) | 44 (77.19) | ||
| Hemarthrosis | 3 (5.0) | 2 (3.3) | 0 (0.00) | 1 (1.75) | ||
| Mucosa | 15 (25.0) | 16 (26.7) | 5 (8.77) | 4 (7.02) | ||
| Skin | 12 (20.0) | 12 (20.0) | 8 (14.04) | 8 (14.04) | ||
| Severity of bleeding, n (%) | .63 | .56 | ||||
| Severe | 47 (78.3) | 49 (81.7) | 49 (85.96) | 51 (89.47) | ||
| Nonsevere | 13 (21.6) | 11 (18.3) | 8 (14.04) | 6 (10.53) | ||
P values refer to McNemar χ2 or Wilcoxon signed-rank test.
Two patients (3.5%) among the matched samples treated with rFVIIa also received ancillary immunoadsorption.
Rates of control for first bleeding episodes by first-line therapy
| Hemostatic agent . | First-line bleeding control . | |
|---|---|---|
| n . | % . | |
| Unmatched samples | ||
| Bypassing agent | 219 | 91.8 |
| FVIIa | 159 | 91.2 |
| aPCC | 60 | 93.3 |
| Replacement therapy | 69 | 69.6 |
| FVIII | 55 | 70.1 |
| DDAVP | 14 | 64.3 |
| PS-matched samples | ||
| Bypassing agent | 60 | 93.3 |
| Replacement therapy | 60 | 68.3 |
| rFVIIa | 57 | 93.0 |
| aPCC | 57 | 93.0 |
| Hemostatic agent . | First-line bleeding control . | |
|---|---|---|
| n . | % . | |
| Unmatched samples | ||
| Bypassing agent | 219 | 91.8 |
| FVIIa | 159 | 91.2 |
| aPCC | 60 | 93.3 |
| Replacement therapy | 69 | 69.6 |
| FVIII | 55 | 70.1 |
| DDAVP | 14 | 64.3 |
| PS-matched samples | ||
| Bypassing agent | 60 | 93.3 |
| Replacement therapy | 60 | 68.3 |
| rFVIIa | 57 | 93.0 |
| aPCC | 57 | 93.0 |
Because the PS matching algorithm selected 41 and 19 patients treated with rFVIIa and aPCC, respectively, to confirm the robustness of the above result, a PS quintiles-adjusted model for treatment comparison was also assessed. The results overlapped completely (OR = 0.20; 95% CI, 0.10-0.46; P < .0001). A sensitivity analysis was also performed to disprove that this positive finding may be attributable to an imbalanced unknown, unmeasured baseline covariate. With respect to the results for the risk of unresolved bleeding episodes, the significant effect of bypassing agents (ie, OR = 0.15; 95% CI, 0.04-0.53) might be altered by an unmeasured baseline covariate with an OR = 2.5 and a prevalence imbalance between the 2 treatment groups of at least 60%. The risks might be also altered with a prevalence imbalance of 50%, but an OR = 3.0, or with a prevalence imbalance of at least 40% but an OR = 3.5.
Baseline characteristics of the 159 patients receiving rFVIIa and the 60 receiving aPCC are reported in Table 1. The baseline covariate significantly different in the 2 unmatched groups was age (P = .02); sex (P = .06) and cause of bleeding (P = .08) were nonsignificant. The rate of bleeding control was similar for rFVIIa treatment: 145 of 159 (91.2%) and aPCC 56 of 60 (93.3%; P = .60). The baseline covariates after PS matching overlapped (Table 2). An equal rate of bleeding control was reported for 53 of 57 patients (93.0%) using both aPCC and rFVIIa, with OR = 1 (95% CI, 0.23-4.44; P = 1; Table 3).
As a further overall sensitivity analysis, a different approach for treatment comparison was also pursued. The impact of first-line hemostatic therapy for patients who were not treated for their very first bleeding episode but were treated for a subsequent episode was recorded in the registry. The findings obtained via PS matching (data not shown) were completely overlapping with the results reported here. To this end, PS models were further matched for cases for which the first bleeding episode was recorded in the registry versus for those for which it was not recorded.
Details of first-line hemostatic therapy (initial dose, initial frequency, number of doses given, and total dose) are provided in Table 4. In general, lower FVIII levels and higher inhibitor titers were observed in patients treated with bypassing agents; however, FVIII levels for all treatment modalities overlapped, suggesting that FVIII level had little influence on treatment decisions.
Types of first-line hemostatic therapy for all first bleeding episodes [median (IQR)]
| Therapy . | n . | Baseline FVIII level, IU/dL . | Baseline inhibitor titer, BU/mL . | Initial dose, μg/kg or U/kg . | Initial dosing interval, hours . | Total doses per patient, n . | Total dose per patient . |
|---|---|---|---|---|---|---|---|
| rFVIIa | 174 | 2.0 | 15.5 | 90 μg/kg | 3 | 12 | 84 mg |
| (0.0-32.0) | (1.0-2765) | (84.71-102.86) | (2-6) | (3-35) | (24-216 mg) | ||
| aPCC | 63 | 1.0 | 18.0 | 66.67 U/kg | 12 | 8 | 30 000 U |
| (0.0-40.0) | (0.1-1700) | (52.63-82.19) | (12-12) | (3-15) | (12 000-56 000 U) | ||
| FVIII | 56 | 3.0 | 7.5 | 52.91 U/kg | 12 | 5 | 20 000 U |
| (0.0-34.0) | (0.8-180) | (40.00-81.97) | (8-12) | (2-10) | (9000-49 500 U) | ||
| DDAVP | 14 | 3.5 | 8.0 | 0.3 μg/kg | 12 | 2.5 | 40 μg |
| (0.0-17.0) | (0.3-200) | (0.3-0.3) | (8-24) | (1-3) | (21-64 μg) |
| Therapy . | n . | Baseline FVIII level, IU/dL . | Baseline inhibitor titer, BU/mL . | Initial dose, μg/kg or U/kg . | Initial dosing interval, hours . | Total doses per patient, n . | Total dose per patient . |
|---|---|---|---|---|---|---|---|
| rFVIIa | 174 | 2.0 | 15.5 | 90 μg/kg | 3 | 12 | 84 mg |
| (0.0-32.0) | (1.0-2765) | (84.71-102.86) | (2-6) | (3-35) | (24-216 mg) | ||
| aPCC | 63 | 1.0 | 18.0 | 66.67 U/kg | 12 | 8 | 30 000 U |
| (0.0-40.0) | (0.1-1700) | (52.63-82.19) | (12-12) | (3-15) | (12 000-56 000 U) | ||
| FVIII | 56 | 3.0 | 7.5 | 52.91 U/kg | 12 | 5 | 20 000 U |
| (0.0-34.0) | (0.8-180) | (40.00-81.97) | (8-12) | (2-10) | (9000-49 500 U) | ||
| DDAVP | 14 | 3.5 | 8.0 | 0.3 μg/kg | 12 | 2.5 | 40 μg |
| (0.0-17.0) | (0.3-200) | (0.3-0.3) | (8-24) | (1-3) | (21-64 μg) |
Thrombotic events in relation to hemostatic therapy were reported in 13 of the 482 patients who experienced a bleeding episode (2.7%), including 2 of 144 patients who were not treated with a hemostatic agent and 11 of the 307 patients treated with a hemostatic agent (3.6%): 7 myocardial infarctions, 1 stroke, 5 venous thromboembolims overall, and 6 myocardial infarctions, 1 stroke and 4 venous thromboembolims in association with hemostatic treatment. The thrombotic events were reported in patients treated with rFVIIa 5 of 174 (2.9%), aPCC 3 of 63 (4.8%), FVIII/DDAVP 0 of 70 (0%), and in 3 cases treatment was not indicated. It is not possible to draw any definite conclusions about the causal relationship between the hemostatic treatment and the thrombotic event from the data available in the registry, except in 1 case where the local investigator reported that the thrombotic event was suspected to be related to treatment with a hemostatic agent (a myocardial infarction in a patient treated with rFVIIa). Furthermore, thrombotic events were significantly associated with mean age (79.4 vs 68.3, P = .0341), but not with underlying clinical conditions (P = .6302).
Overall mortality among patients in whom treatment was initiated was 66 of 338 (19.5%). Among patients who received ancillary therapy alone, 6 of 31 (19.4%) died and survival at years 1 to 4 remained steady at 83%. Overall, 29 of 174 (16.7%) who were treated for a first bleeding episode with rFVIIa died, with a survival of 84%, 82%, 79%, and 72% at years 1 to 4, respectively. Similarly, 12 of 63 (19.0%) patients treated with aPCC died, with 81% surviving at 1 year and 76% surviving at each of years 2 to 4. A total of 19 of 56 (33.9%) patients treated for a first bleeding episode with FVIII died, with survival at years 1 to 4 of 74%, 70%, 57%, and 44%. No deaths were reported among the 14 patients treated with DDAVP. A total of 16 of 482 patients who experienced at least 1 bleeding episode and 10 of 307 patients treated with hemostatic therapy died as a result of bleeding (mortality, 3.3%). Four of 16 deaths occurred on the first day of therapy. The median time from therapy to death was 23 days. Among the patients who died after hemostatic treatment for the first bleeding episode, 4 received rFVIIa, with a median of 19 (1, 15, 23, and 27) days between treatment and death, 3 received aPCC a median of 16 (1, 16, and 163) days previously, and 3 were treated with FVIII a median of 25 (23, 25, and 184) days before death. An anamnestic response to treatment of the first bleeding episode was reported in 6 of 63 patients (9.5%) treated with aPCC, 15 of 55 (27.2%) treated with FVIII, and 3 of 14 (21.4%) treated with DDAVP. No information on the kinetics of the inhibitor in these cases was recorded. There were no anaphylactic or allergic reactions reported.
Discussion
The EACH2 registry provides information on routine clinical practice in Europe in the management of this rare but serious hemorrhagic condition. The study is the largest observational dataset reported to date and more than doubles the number of patients in a single cohort previously reported in the literature. The primary aim of this study was to report how the first bleeding episode was managed; the treatment of subsequent bleeds are not discussed in terms of comparison because the outcome may have been influenced by the treatment of, and response to, first bleeds. A total of 307 patients were included in this analysis, and 288 patients had information on the main outcome (bleeding controlled or not controlled) recorded.
Two therapeutic interventions to control bleeding in patients with AHA are available: bypassing agents (rFVIIa and aPCC) and strategies to increase FVIII levels (FVIII concentrate and DDAVP). Although not administered by any of the participating centers, in the past prothrombin complex concentrates were also used to treat patients with classic hemophilia and FVIII inhibitors and sporadically in AHA, with a similar efficacy and safety profile in both conditions18 ; however, their efficacy appears to be lower than either rFVIIa or aPCC, and their use is not recommended in current guidelines for the treatment of AHA.10 Bypassing agents are recommended as first-line therapy because of their rapid action and high level of effectiveness.10-12 Prospective randomized trials comparing the efficacy of these agents have not been carried out to date, and it is very unlikely that an adequately powered study would be feasible. No high-level evidence is therefore available for the use of either product. The dosage is largely based on experience with the management of patients with FVIII alloantibody inhibitors in congenital hemophilia and is generally based on the clinical assessment.
The EACH2 registry is a representative cohort of patients, which largely confirms existing baseline data.1-3,5,6,19-24 Clinical bleeding at presentation was observed in 96.2% of patients and was severe in 69.5%. Severity of bleeding is widely variable, and it is recognized that many patients do not require hemostatic treatment. The finding that 30% of patients with bleeding in EACH2 did not receive hemostatic therapy is in agreement with previous reports.3 FVIII level and inhibitor titer were not related to the presence of bleeding, a requirement for hemostatic therapy or severity of bleeding as previously reported.13 Although some patients do not require hemostatic treatment, in many cases bleeding in AHA is an emergency.3,19 In our cohort, 194 patients (38.7%) either did not exhibit any bleeding or did not require hemostatic therapy at presentation. In those that did require hemostatic therapy, bleeding was controlled in 86.7% (269 of 307) by the initial therapy. rFVIIa was the most commonly used hemostatic treatment, administered in 174 of 307 patients (56.6%).
The effectiveness of bypassing agents compared with strategies to raise FVIII levels on bleeding resolution was assessed. In the unmatched population, the bleeding control with the first-line therapy (primary end point) was obtained in 201 patients (91.8%) treated with bypassing agents compared with 48 (69.6%) treated with replacement therapy (P < .003). This substantial difference was confirmed after PS matching in 60 pairs and strongly supports the recommended use of bypassing agents.10-12 The control rate of bleeding episodes with bypassing agents as first-line therapy in our registry is similar to that reported by Sumner et al with rFVIIa (91.8 vs 95%, respectively).9 The experience with aPCC in AHA derives from single-center cohort studies composed of relatively few patients. The control of bleeding episodes varied between 76% and 100% depending on the severity of hemorrhage.20,25-27 These data are not comparable with the data relating to rFVIIa because of differences in patient characteristics and the type and severity of bleeding episodes.
No studies have compared rFVIIa and aPCC in AHA, although studies in congenital hemophilia with inhibitors suggest similar efficacy.28,29 This PS-matched analysis was carried out in 57 matched pairs and provides the only currently available comparison of rFVIIa and aPCC in AHA. Bleeding control was the same in the PS-matched cohorts, supporting the view that these agents have a very similar hemostatic efficacy in AHA. The results of the registry, therefore, confirm that bypassing agents are more efficacious than FVIII or DDAVP and should be the agents of choice for the first-line therapy. rFVIIa versus aPCC have similar efficacy.
The EACH2 database provides important information about thrombotic adverse events associated with hemostatic agents used to treat bleeding episodes in AHA. Adverse events were seen at similar rates with both rFVIIa (2.9%) and aPCC (4.8%) but not with FVIII or DDAVP. Two patients experienced thrombotic events in the absence of hemostatic therapy. Sumner et al have reported an incidence of thrombosis of 6.5% associated with rFVIIa use in AHA.9 It is possible that the safety information may be underestimated, as adverse events are infrequently published as part of case reports. On the other hand, a thrombotic event must take into consideration many other factors related to thrombotic risk, possibly independent of hemostatic agents administered. Previous data on thrombotic events in patients with AHA treated with aPCC are lacking, and the agent is reported to be well tolerated.20,25-27 In the papers evaluating the safety of aPCC and rFVIIa in patients with labeled indications (the majority are patients with hemophilia A and inhibitors), the incidence of thrombotic events was similar, at 4 per 100 000 infusions.30,31
The higher rate of arterial and venous thrombosis in AHA compared with congenital hemophilia with inhibitors31 may be attributable to the age of the patients studied (median age, 74 years), the likelihood of comorbidities, and the presence of acquired thrombotic risk factors. The finding of a significant number of thrombotic events highlights the importance of avoiding unnecessary treatment of patients with mild or superficial bleeding and supports the view of consensus guidelines that rFVIIa at a dose of 270 μg/kg, commonly used in congenital hemophilia with inhibitors, may not be appropriate in AHA.32 The registry reports a bleeding mortality rate of 3.3%, much lower than that reported for the United Kingdom registry (8%).3 This difference may reflect improved awareness of the clinical condition and the availability of effective hemostatic agents. It is also possible that the difference may result from the way in which the cohorts were collected.
Some limitations to the EACH2 registry dataset exist. Considering the total population of the participating countries (374 million) and the estimated incidence of AHA (∼ 1.5 per 106 population/year), as reported in the United Kingdom registry,3 the estimated number of cases among this population that might have been expected during the 6 years of data collection was approximately 3360. This means that only 14.9% of the potentially eligible patients (501 of 3360) were enrolled; however, this rate of success for a registry is a significant achievement. The low enrollment may be explained by the low number of participating centers within the recruitment area or may reflect the true incidence of critical cases that may differ in different geographic or ethnic backgrounds. The low enrollment and unrestricted patient management are important limits to the dataset. Participating centers entered consecutive patients; therefore, some forms of reporting bias were reduced. However, most participating centers were specialist referral centers; therefore, some level of referral bias is likely, and the population may reflect a more severely affected cohort than that reported in the United Kingdom.3 The registry did not try to influence treatment directly, although links to national treatment guidelines were provided. This means that clinicians managed patients according to their routine protocols; and hence, management was highly variable.
As for all observational sources of data, one major issue is always the lack of randomization and therefore potential selection bias when addressing any treatment comparison. PS methodology is a useful tool to reduce selection bias in observational studies15,16 and has already been used in many therapeutic fields.33-36 Treatment comparisons in such contexts are still prone to residual bias, which PS methods cannot take into account, however; and although sensitivity analysis can overcome residual bias by addressing its extent, these attempts to assess treatment effectiveness should be considered exploratory in nature.
In conclusion, this study reports both the largest collection of bleeding episodes recorded in AHA and the most rigorous analysis of treatment outcome. The data show that the optimal treatment of bleeding in AHA is composed of bypassing agents, which can be expected to resolve bleeding in more than 90% of cases. The data support caution in the use of bypassing agents in the typical AHA population because of an association with both arterial and venous thrombotic events.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank our collaborators in the participating centers for data acquisition and entry, Francesco de Cataldo for critical reading of the manuscript and discussion, and Parexel International GmbH (Berlin, Germany) for technical and registry database support.
The registry was supported by Novo Nordisk Region Europe A/S, Zurich, Switzerland (unrestricted grant). Editorial assistance was provided by Physicians World Europe GmbH (Mannheim, Germany), supported by Novo Nordisk Heath Care AG, Zurich, Switzerland.
Authorship
Contribution: F.P. was primarily responsible for the statistical analyses; F.B. and F.P. drafted the manuscript; and all authors participated in definition of data fields to be collected in the registry, developed the electronic case report form, and reviewed, revised, and approved the manuscript.
Conflict-of-interest disclosure: F.B. has received honoraria directly from Bayer, Baxter, Grifols, and Novo Nordisk. P.C. has served as a consultant for Novo Nordisk, Baxter Healthcare, CSL Behring, and Inspiration Pharmaceuticals and has received honoraria directly from Novo Nordisk, Baxter Healthcare, Bayer, CSL Behring, and Inspiration Pharmaceuticals. A.H.-K. has served as a consultant for Novo Nordisk, Bayer, and Pfizer. P.K. has served as a consultant, received research and travel funding, and has been a member of advisory committees for Novo Nordisk, Baxter, Archemix, and Ablynx. H.L. has served as consultant for Novo Nordisk and Baxter Healthcare and has received honoraria for lecturing from Novo Nordisk. P.M. has served as consultant and has been a member of advisory committees for Novo Nordisk. L.N. has received honoraria for lecturing and participation in advisory boards for Baxter, Novo Nordisk, and Pfizer. F.P. has served as a consultant for Novo Nordisk. L.T. has served as a consultant for Ferring and Novo Nordisk and has received honoraria directly from them.
A complete list of the EACH2 registry contributors appears in the online supplemental Appendix.
Correspondence: Francesco Baudo, Thrombosis and Hemostasis Unit, Ospedale Niguarda, Piazza Ospedale Maggiore 3, I-20162 Milan, Italy; e-mail: md9821@mclink.it.