Abstract
Fanconi anemia (FA) is a rare bone marrow failure disorder with defective DNA interstrand crosslink repair. Still, there are FA patients without mutations in any of the 15 genes individually underlying the disease. A candidate protein for those patients, FA nuclease 1 (FAN1), whose gene is located at chromosome 15q13.3, is recruited to stalled replication forks by binding to monoubiquitinated FANCD2 and is required for interstrand crosslink repair, suggesting that mutation of FAN1 may cause FA. Here we studied clinical, cellular, and genetic features in 4 patients carrying a homozygous 15q13.3 micro-deletion, including FAN1 and 6 additional genes. Biallelic deletion of the entire FAN1 gene was confirmed by failure of 3′- and 5′-PCR amplification. Western blot analysis failed to show FAN1 protein in the patients' cell lines. Chromosome fragility was normal in all 4 FAN1-deficient patients, although their cells showed mild sensitivity to mitomycin C in terms of cell survival and G2 phase arrest, dissimilar in degree to FA cells. Clinically, there were no symptoms pointing the way to FA. Our results suggest that FAN1 has a minor role in interstrand crosslink repair compared with true FA genes and exclude FAN1 as a novel FA gene.
Introduction
Fanconi anemia (FA) is characterized by chromosome breakage, congenital malformations, pancytopenia, and cancer susceptibility.1 FA is a rare disease with a carrier frequency of 1:65 to 1:209.2,3 FA cells are hypersensitive to DNA interstrand crosslinking (ICL) drugs, such as mitomycin C (MMC) and diepoxybutane (DEB), and the diagnostics relies on an excess chromosome fragility after in vitro exposing patients' cells to these agents. There are at least 15 independent FA subtypes, each resulting from mutation of a distinct FA gene.4-7 However, a minority of FA patients remain unassigned, suggesting the existence of additional FA genes. Recently, 4 groups reported that FA nuclease 1 (FAN1) is a good candidate for a novel FA gene.8-11 The reason is that FAN1 is recruited to stalled replication forks by binding to monoubiquitinated FANCD2, and its nuclease activity is required for ICL repair. Transient depletion of FAN1 in human transformed fibroblasts led to increased MMC-induced chromosome breakage rates. Consequently, all 4 groups suggested that FAN1 mutations may cause FA.8-11
FAN1 maps to 15q13.3. Heterozygous 15q13.3 microdeletion has been associated with a variety of symptoms, including mental retardation, epilepsy, psychiatric disease, autism spectrum disorders, muscular hypotonia, and dysmorphic facial features. Penetrance of the microdeletion disorder is variable and encompasses severely affected patients to normal persons.12 Apart from FAN1, 6 additional genes are located in 15q13.3 (ARHGAP11B, MTMR10, TRPM1, KLF13, OTUD7A, and CHRNA7). Here we studied 4 patients with homozygous 15q13.3 microdeletion12,13 to clarify whether lack of FAN1 may lead to FA.
Methods
Clinical features and blood samples were obtained from 4 homozygous 15q13.3 microdeletion patients (MD1-MD4) all previously diagnosed by array comparative genomic hybridization and quantitative PCR. Two of these patients (MD1 and MD2) have been mentioned before.12,13 Lack of FAN1 was confirmed at the gene level by PCR and at the protein level by Western blotting. The PCR primers used to amplify the 3′ and 5′ flanking regions of FAN1 were as follows: ex1 forward, 5′AGGGTTGTCTCCTCGTTACAGGA3′; ex1 reverse, 5′GCTGAATCACTTTGGCCAGG3′; ex15 forward, 5′CTTCCTAAAACCTGCTGGAGG3′; and ex15 reverse, 5′AATGTACTGACCGTGTGCTCA3′. PCR, Western blot analysis, survival assays, and chromosome breakage assays were performed as described elsewhere.3,14-18 FAN1-monospecific antibody was kindly provided by Dr John Rouse (Dundee, United Kingdom) and used at 1:500 dilution. A total of 27 genetically unassigned FA cell lines had previously been excluded from belonging to any of the reported 15 FA complementation groups. This study was ethically approved by the Universitat Autònoma de Barcelona Institutional Review Board. Informed consent was obtained from all families in accordance with the Declaration of Helsinki.
Results and discussion
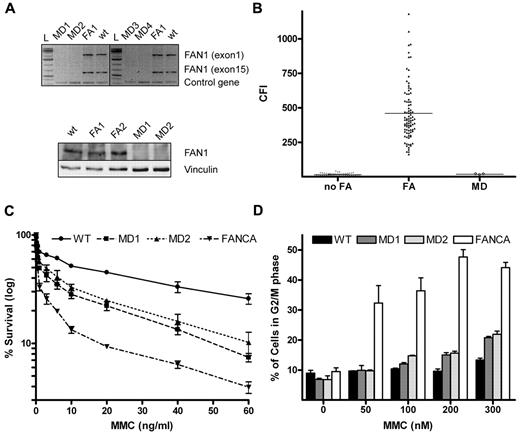
Study of FA candidate genes may enable the final classification of unassigned FA patients. Four recent studies have proposed FAN1 as a putative FA gene.8-11 Here we studied 4 patients (MD1-MD4) with homozygous 15q13.3 microdeletion to clarify whether FAN1 deficiency leads to features consistent with FA. Two of these patients (MD1 and MD2) have previously been mentioned in unrelated reports12,13 and the other 2 are newly recognized siblings, detected by array comparative genomic hybridization (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Considering that 15q13.3 microdeletion may have 3 different extensions,19 we aimed to corroborate homozygous absence of the FAN1 gene by failure to PCR amplify its first (exon 1) and last (exon 15) exon from genomic DNA. DNA of 2 additional lymphoblastoid cell lines (LCLs), from a normal person and from a FANCA-deficient patient, served as controls. As shown in Figure 1A top panel, FAN1 PCR products are absent in all MD patients, confirming that all 4 MD patients have biallelic deletion of the entire FAN1 gene. Moreover, lack of FAN1 expression was confirmed by FAN1 immunoblotting. Clearly, the FAN1-specific band was missing in the 2 available LCLs from MD patients (MD1 and MD2), whereas FAN1 was readily detected in the control LCLs (Figure 1A bottom panel).
Absence of FAN1 and FA cellular phenotype in MD patients. (A) Fragments amplified by PCR corresponding to exons 1 and 15 of the FAN1 gene were observed using DNA templates from a healthy person (WT) and a FANCA-deficient FA patient included as controls, whereas they were absent when templates from microdeletion patients (MD1 to MD4) were used, confirming the biallelic deletion of FAN1 in the MD patients (top panel). Immunoblotting against FAN1 protein revealed a FAN1-specific band in WT and FA (FA1 and FA2) LCL that failed to be detected in MD1 and MD2. (bottom panel). (B) Dot plot of CFI showing individual values and average (solid line) of DEB-induced chromosome breakage from non-FA (n = 56), FA (n = 90, excluding mosaics) and MD (n = 4) persons. The CFI values of all MD patients ranged within the non-FA population. (C) Mild sensitivity of MD LCL to MMC on survival assay. The graph shows intermediate sensitivity to MMC of MD1 and MD2 compared with the highly sensitive FA (FANCA) cell line. (D) Near-normal sensitivity of MD LCL to MMC on cell cycle analysis. The graph plots the percentage of cells in G2/M phase after exposure to increasing concentrations of MMC for 72h. A WT and an FANCA cell lines were included as controls.
Absence of FAN1 and FA cellular phenotype in MD patients. (A) Fragments amplified by PCR corresponding to exons 1 and 15 of the FAN1 gene were observed using DNA templates from a healthy person (WT) and a FANCA-deficient FA patient included as controls, whereas they were absent when templates from microdeletion patients (MD1 to MD4) were used, confirming the biallelic deletion of FAN1 in the MD patients (top panel). Immunoblotting against FAN1 protein revealed a FAN1-specific band in WT and FA (FA1 and FA2) LCL that failed to be detected in MD1 and MD2. (bottom panel). (B) Dot plot of CFI showing individual values and average (solid line) of DEB-induced chromosome breakage from non-FA (n = 56), FA (n = 90, excluding mosaics) and MD (n = 4) persons. The CFI values of all MD patients ranged within the non-FA population. (C) Mild sensitivity of MD LCL to MMC on survival assay. The graph shows intermediate sensitivity to MMC of MD1 and MD2 compared with the highly sensitive FA (FANCA) cell line. (D) Near-normal sensitivity of MD LCL to MMC on cell cycle analysis. The graph plots the percentage of cells in G2/M phase after exposure to increasing concentrations of MMC for 72h. A WT and an FANCA cell lines were included as controls.
To check whether FAN1 deficiency leads to DEB-induced chromosome fragility,20,21 we performed DEB tests on an LCL from patient MD1 and on blood T cells from patients MD2 to MD4. Chromosome breakage rates were quantified with the recently described chromosome fragility index (CFI)17 and the results compared with our historical database.17 Clearly, the CFI of all MD patients fell into the range of the non-FA group (Figure 1B). Similar results were obtained with MMC (data not shown).
We next tested the survival of the 2 available MD LCLs in response to MMC. MD1, MD2, a wild-type and a FANCA LCL were challenged with 0 to 100 ng/mL of MMC. Based on LD50 values, the MD cell lines showed mild sensitivity to MMC: whereas FANCA-deficient cells were more than 30-fold more sensitive to MMC than WT cells, MD1 and MD2 cells were, on average, 5-fold more sensitive to MMC than WT cells (Figure 1C). Silencing of the FAN1 gene by siRNA was previously shown to impair ICL repair, leading to hypersensitivity of cells to ICL. However, this hypersensitivity was also intermediate compared with mRNA depletion of authentic FA genes, such as FANCA, FANCD2, or FANCJ.8-10 This set of data suggests that the cellular response of FAN1-deficient cells to MMC is not fully functional but not impaired as in FA.
To further study the FA pathway in the absence of FAN1, cell cycle distributions of FAN1-deficient cell lines were analyzed by flow cytometry.22 Exposure to increasing concentrations of MMC for 72 hours resulted in G2 arrest at low MMC concentrations in FA-A LCL, whereas G2 arrest was very mild in the MD samples (Figure 1D), compatible with the mild sensitivity to MMC shown before. These results are consistent with a recent report on ΔFAN1-DT40 cells showing that FAN1 protects cells against ICL agents in a pathway, which is not epistatic with the FA pathway and that FAN1 assumes in the processing of ICL only a secondary role or functions independently of the FA pathway.23 We finally analyzed FAN1 protein expression levels in 27 cell line from unassigned FA patients by Western blotting. All of the unassigned FA cell lines expressed FAN1 protein at control levels, suggesting that none of these patients had major deficiency of this protein (supplemental Figure 2).
To assess the hematologic impact of FAN1 deficiency, we obtained clinical data and hemograms of MD2, MD3, and MD4. Normal hematology had earlier been reported for MD1.12 As shown in Table 1, MD patients do not present with anemia, bone marrow failure, skin pigmentation anomalies, or FA-typical malformations, such as skeletal abnormalities of the upper limbs. Three of the MD patients (MD1, MD3, and MD4) showed microsomy and microcephaly, which is often seen in FA patients but also in other syndromes with defective processing of stalled replication forks, such as Seckle and Bloom syndromes and can be regarded as common symptoms of patients with DNA repair defects.24 Yet we cannot conclude for certain that microcephaly and microsomy found in MD patients are caused by FAN1 deficiency because 6 additional genes are included in the 15q13.3 region. However, it is tempting to speculate that this is the case as FAN1 directly interacts with FANCD2, and 90% of patients with FANCD2 mutations have microcephaly.15
Patient characteristics
| Patient no. . | Age, y . | Nationality . | Clinical features . | Hematology . | Chromosome fragility . | Sensitivity to MMC . | G2/M block . | Reference . |
|---|---|---|---|---|---|---|---|---|
| MD1 | 11 | United States | Visual impairment, hypotonia, areflexia, absent language, epilepsy, microsomy, and microcephaly | Normal | Negative | Mild | Mild | 13 |
| MD2 | 6 | France | Hypotonia, severe developmental delay; rod–cone dystrophy, epilepsy, and autistic features | Normal | Negative | Mild | Mild | 12 |
| MD3 | 1 | France | Severe developmental delay, visual impairment, microsomy, and microcephaly | Normal | Negative | ND | ND | Present study |
| MD4 | 3 | France | Severe developmental delay, absent language, visual impairment, microsomy, and microcephaly | Normal | Negative | ND | ND | Present study |
| Patient no. . | Age, y . | Nationality . | Clinical features . | Hematology . | Chromosome fragility . | Sensitivity to MMC . | G2/M block . | Reference . |
|---|---|---|---|---|---|---|---|---|
| MD1 | 11 | United States | Visual impairment, hypotonia, areflexia, absent language, epilepsy, microsomy, and microcephaly | Normal | Negative | Mild | Mild | 13 |
| MD2 | 6 | France | Hypotonia, severe developmental delay; rod–cone dystrophy, epilepsy, and autistic features | Normal | Negative | Mild | Mild | 12 |
| MD3 | 1 | France | Severe developmental delay, visual impairment, microsomy, and microcephaly | Normal | Negative | ND | ND | Present study |
| MD4 | 3 | France | Severe developmental delay, absent language, visual impairment, microsomy, and microcephaly | Normal | Negative | ND | ND | Present study |
MMC indicates mitomycin C; and MD, microdeletion.
Even though LCLs with total FAN1 deficiency reveal mild sensitivity to MMC on some assays, normal expression of FAN1 in 27 unassigned FA cell lines, the lack of DEB- or MMC-induced chromosome fragility, and the absence of hematologic defects or FA-archetypal malformations exclude FAN1 as being an FA gene.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr B. C. Bittel (University of Missouri–Kansas City School of Medicine, Kansas City, MO) for providing an LCL from patient MD1 and Dr J. Rouse (University of Dundee, Dundee, United Kingdom) for sharing his anti-FAN1 antibody.
The laboratory of J.S. was supported by the Generalitat de Catalunya (SGR0489-2009), the Institut Català de Recerca i Estudis Avançats-Academia award, the Spanish Ministry of Science and Innovation (projects CB06/07/0023, and SAF2009-11936), and the European Regional Development Funds. Centre for Biomedical Network Research on Rare Diseases is an initiative of the Instituto de Salud Carlos III.
Authorship
Contribution: J.P.T., L.B.M., R.P., M.B., and B.S. performed experiments and helped write the manuscript; J.A. and M.H. provided essential research materials and clinical data and performed experiments; D.S. designed experiments and provided essential research materials; and J.S. coordinated and supervised the study, designed experiments, and wrote the paper with the help of L.B.M.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jordi Surrallés, Genome Instability and DNA Repair Group, Department of Genetics and Microbiology, Universitat Autònoma de Barcelona, Campus de Bellaterra S/N, 08193, Bellaterra, Barcelona, Spain; e-mail: jordi.surralles@uab.es.
References
Author notes
J.P.T. and L.B.M. contributed equally to this study.