In this issue of Blood, Jaako et al1 and Payne et al2 provide important preclinical validation that the essential amino acid leucine can ameliorate defective erythropoiesis in murine and zebrafish models of Diamond-Blackfan anemia, respectively.
Diamond-Blackfan anemia (DBA) is a rare genetic disease resulting from sporadic mutations and occasional autosomal-dominant inheritance. Almost uniformly, patients manifest in the newborn period with a macrocytic anemia, occasional multilineage cytopenias, variable developmental abnormalities, and a propensity for cancer.3,4 Underlying mutations in 9 genes encoding ribosomal proteins (RPs) have been identified to date, in aggregate accounting for about half the diagnosed cases. In the wake of these discoveries, much has been learned about the consequences of dysregulated ribosome biogenesis, in particular the tissue-specific effects of mutations in RPS19, the most common and earliest-identified genetic lesion in DBA.5 The mature ribosome is a macromolecular complex dependent on the tightly balanced availability of RNA and ribosomal proteins. Haploinsufficiency of individual RPs, genetic as in the case of DBA patients or synthetic in the case of the respective mouse and zebrafish models, disrupts that stoichiometric relationship. The resulting negative effect on ribosome biogenesis spells vulnerability, especially for tissue functions with high demand on translational efficiency, such as erythropoiesis.6
For the hematopoietic manifestations of DBA, stem cell transplantation is curative but limited by donor availability and justified concerns over the procedure-related risks. Patients, therefore, receive symptomatic treatment with red blood cell transfusions and rely on corticosteroids, to which the majority are responsive.3 Drug-induced or spontaneous remissions are seen in up to 20% of patients, but the prospect of a lifetime of steroid side effects looms large and the search for alternative, steroid-sparing treatments has been elusive. A case report several years ago generated excitement among patients and practitioners when a young girl, refractory to steroid treatment and given supplements of the amino acid leucine, experienced symptomatic improvement in erythropoiesis.7 Prior in vitro work had already suggested that leucine could be effective for DBA caused by mutations in several known genes. L-leucine is thought to boost translation by enhancing the activation of translation initiation factors that regulate mRNA binding to the ribosomal complex and by a specific up-regulation of ribosome biosynthesis through the mTOR-regulated, ribosomal protein S6 kinase.5,8 However, clinical trials for leucine treatment of DBA have been slow to materialize, in spite of this tantalizing case report and a presumptive understanding of the cellular mechanism. Meanwhile, the field was heavily focused on the design of faithful genetic models of DBA. Unfortunately, generation of an Rps19 knockout mouse itself proved a challenge, with conventional strategies alternately yielding animals that were nearly asymptomatic or outright embryonic-lethal.6,9 Here, Jaako and colleagues successfully applied an approach based on the inducible knockdown of Rps19 via short hairpin RNA interference,1 incidentally a similar strategy taken by Payne et al for some of their studies (see figure).2 On induction, the animals develop several characteristic features of DBA, including macrocytic anemia with decreased hemoglobin values and erythrocyte numbers.9 Remarkably, simultaneous L-leucine supplementation significantly ameliorated these effects even though 2 downstream mediators of the mTOR kinase, 4E-BP1 and Rps6, were surprisingly unaffected.8 The critical reader will note that this approach hardly replicates the chronic disease state in patients. Yet, the experiments provide critical proof of concept to motivate further study of L-leucine and validate the model as a tool to examine the disease pathogenesis itself.
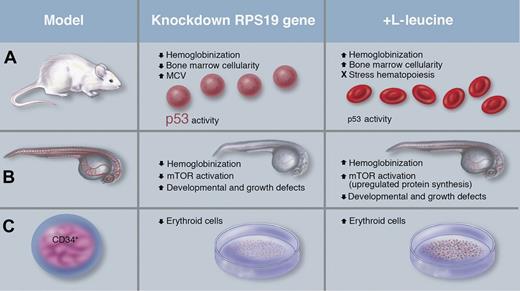
Key findings in 3 systems of inducible knockdown of the Rps19 gene. (A) The doxycycline-inducible mouse described by Jaako et al exhibited reduced hemoglobinization, an increase in mean corpuscular volume (MCV), decreased RBC counts, a decrease in bone marrow cellularity, and evidence of increased p53 activity. Simultaneous administration of L-leucine and doxycycline induction of Rps19 knockdown improved these symptoms. (B) Payne et al relied on morpholino-injected zebrafish embryos that displayed reduced hemoglobin and mTOR activation, alongside defects in development and growth, which were partially ameliorated with leucine treatment. (C) Knockdown of RPS19 in human CD34+ cells by Payne et al resulted in differentiation of fewer erythroid cells. In vitro culture with L-leucine resulted in gains in erythroid cell numbers. Professional illustration by Marie Dauenheimer.
Key findings in 3 systems of inducible knockdown of the Rps19 gene. (A) The doxycycline-inducible mouse described by Jaako et al exhibited reduced hemoglobinization, an increase in mean corpuscular volume (MCV), decreased RBC counts, a decrease in bone marrow cellularity, and evidence of increased p53 activity. Simultaneous administration of L-leucine and doxycycline induction of Rps19 knockdown improved these symptoms. (B) Payne et al relied on morpholino-injected zebrafish embryos that displayed reduced hemoglobin and mTOR activation, alongside defects in development and growth, which were partially ameliorated with leucine treatment. (C) Knockdown of RPS19 in human CD34+ cells by Payne et al resulted in differentiation of fewer erythroid cells. In vitro culture with L-leucine resulted in gains in erythroid cell numbers. Professional illustration by Marie Dauenheimer.
The related study by Payne et al relied on a different model system but reinforces key observations, particularly that L-leucine treatment provides general benefit and improves erythropoietic function (see figure). The investigators used a zebrafish model in which morpholinos were injected into embryos to knock down expression of rps19. Rewardingly, the zebrafish morphants recapitulated some characteristic DBA developmental defects, including stunted growth, with defects in the cranium, eye, and heart, which were rescued by L-leucine treatment. Similarly, L-leucine supplementation significantly mitigated the anemia phenotype and improved red cell hemoglobinization. These effects coincided with an increase in phosphorylation of the mTORC1 target S6K1 (but not 4E-BP1) and a substantial increase in phosphorylation of one of its downstream effectors, S6, in the caudal hematopoietic tissue, a site of hematopoietic progenitor development and differentiation. In turn, phosphorylation was blocked by rapamycin, a widely used antagonist of the mTOR pathway. Further, when the investigators studied RPS19-knockdown human CD34+ cells, they observed improvements in erythroid expansion after L-leucine supplementation in culture. These findings, along with those by Jaako et al and the patient case reported by Pospisilova et al,7 would seem to make a compelling case for testing leucine in DBA patient trials.
A note of caution is warranted, however, given that the hematopoietic defect in DBA, and its rescue by L-leucine, appear to involve the cellular p53 response.9,10 When tested in L-leucine–treated and –untreated Rps19-deficient mice, the expression of p53 targets, Phlda3 and Ccng1, was significantly reduced in myeloid progenitors as well as erythroid progenitors, along with down-regulation of the cell-cycle inhibitor Cdkn1a (p21) in the latter. While not fully clarified to date, it is thought that the RPS19 haploinsufficiency in DBA alters the balance and subcellular distribution of other ribosomal proteins, now free to scavenge the E3 ubiquitin ligase, murine double minute (MDM)2 (HDM2 in human). This reduces ubiquitination and proteasomal degradation of p53, increasing its cellular pools and prompting hematopoietic suppression.5,10 With its pivotal role in simultaneously assuring DNA repair integrity and genome stability, p53 itself may make a challenging target for therapy. Accumulation of p53 as a key event on the path to bone marrow failure is not unique to “ribosomopathies” such as DBA,5 but seemingly shared with other cancer predisposition syndromes, including Fanconi anemia. Consequently, the challenge for developing effective drugs may lie in segregating the downstream targets of p53 that promote bone marrow failure from those that “guard the genome” and prevent cancer—even for a seemingly innocuous supplement like L-leucine.4 Along those lines, further gains in understanding how steroids work in these models may help identify specific steroid-responsive targets and to rationally design drugs that would avoid debilitating long-term side effects.
In sum, the present work should raise cautious hope in patients and providers. While direct mechanistic insight is only emerging, these important studies validate a potential role for L-leucine and provide important clues to a steroid-sparing, transplant-free treatment of DBA.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal