In this issue of Blood, Hoeppner et al create an extremely valuable model of vascular permeability that is amenable to high throughput chemical screening as well as genetic analysis.1
Vascular permeability is a major cause of morbidity and mortality in human disease. The edema of glioblastoma multiforme, which increases intracranial pressure, is due to tumor vasculature leakage. Cerebral malaria, which also causes death, is characterized by heightened vascular permeability, and mortality is associated with high levels of Angiopoietin-2 (Ang-2), a major mediator of vascular permeability.2 Tumors with high levels of Ang-2 and vascular permeability are associated with a poor prognosis, possibly because vascular leak causes increased tumor hypoxia due to ineffective perfusion, and hypoxia promotes increased secretion of vascular endothelial growth factor, epithelial mesenchymal transformation, and increased stem cell characteristics. Finally, bacterial sepsis is a major cause of death in humans, and is poorly responsive to pressors that increase blood pressure, because increasing blood pressure merely pushes fluid out of the blood vessels, leading to the phenomenon called third spacing. Given that recent trials for sepsis-reducing agents have met little success, a greater understanding of vascular permeability is required.3
Currently, the gold standard of vascular permeability studies is the Miles Blue assay, in which a dye, Miles Blue, is infused into mice with various pathologic conditions. The quantity of dye that leaks into the resulting tissue is assessed spectrophotometrically. This type of assay is laborious and not amenable to high throughput analysis. In addition, the mouse system makes genetic analysis of vascular permeability difficult, and requires not only the time-consuming process of knocking out a gene, but also that the mice survive the knockout. Here, Hoeppner et al have devised an innovative zebrafish model to study vascular permeability.1 In this transgenic model, heat shock is used to activate the induction of VEGF in a highly reproducible fashion. Using this model, vascular permeability can be divided into basal vascular permeability (likely reflecting vascular tone), acute vascular permeability, and chronic vascular permeability. Chronic vascular permeability likely represents the phenomena seen in human cancer and chronic infections.
VEGF, which was initially described as vascular permeability factor (VPF), activates multiple signaling pathways, mostly downstream of activation of VEGFR2. The ability to separate the promigratory pathways that stimulate endothelial chemotaxis, versus the vascular permeability function, is clinically relevant. Notably, one of the major side effects of the anti-VEGF factor bevacizumab (Avastin), is hypertension, and often limits use of bevacizumab. In the article by Hoeppner et al, the authors describe a positive role of phospholipase Cγ in promoting vascular permeability, which is opposed by phospholipase Cβ3. The role of phospholipase Cβ3 in opposing vascular permeability is confirmed in knockout mice for phospholipase Cβ3. The opposing roles of these phospholipases appear to be at the level of intracellular calcium. An additional upstream factor may be increased reactive oxygen. Of interest, mice that have an endothelial deficiency of NADPH oxidase 2 (Nox2) or the classic transient receptor potential channel 6 are protected from lung ischemia-reperfusion injury, and reactive oxygen was shown to induce phospholipase Cγ, placing reactive oxygen species upstream of phospholipase Cγ.4
The availability of these zebrafish has the potential to revolutionize the study of vascular permeability. First, these models will allow high throughput screening of compounds to assess their impact on vascular permeability. Second, it can be used to assess whether all forms of vascular permeability use the same pathways. For instance, does Ang-2, one of the major instigators of vascular permeability, induce phospholipase Cγ or repress phospholipase Cβ3?
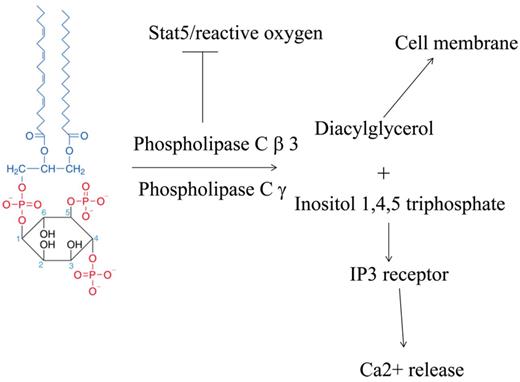
Third, it allows the assessment of antiangiogenic agents on vascular permeability. Do hemangiomas of infancy, the classic manifestation of vascular permeability, have high levels of phospholipase Cγ and/or low levels of phospholipase Cβ3? Do agents that inhibit hemangioma growth, such as NADPH oxidase inhibitors (fulvenes, gentian violet) and β blockers, reverse the imbalance between phospholipase Cγ and phospholipase Cβ3 (angiogenic switch)5-8 ? Finally, both phospholipase Cγ and phospholipase Cβ3 have similar enzymatic activity, but phospholipase Cβ3 has been shown to be a tumor suppressor, because of nonenzymatic functions of phospholipase Cβ3, namely activation of the stem cell factor stat5 (see figure).9 It is the unopposed action of phospholipase Cγ that likely accounts for the angiogenic switch to increased vascular permeability.
Both phospholipase Cβ3 and phospholipase Cγ have similar enzymatic activities in cleavage of PIP2 into diacylglycerol and Ins (1,2,4) triphosphate. Diacylglycerol activates canonical protein kinase C, while Ins (1,2,4) triphosphate binds to its receptor IP3R, resulting in calcium release. Loss of phospholipase Cβ3 results in activation of stat5 and possibly reactive oxygen in the face of unopposed phospholipase Cγ, resulting in proliferation and increased vascular permeability. Structure of PIP2 from Wikipedia.10
Both phospholipase Cβ3 and phospholipase Cγ have similar enzymatic activities in cleavage of PIP2 into diacylglycerol and Ins (1,2,4) triphosphate. Diacylglycerol activates canonical protein kinase C, while Ins (1,2,4) triphosphate binds to its receptor IP3R, resulting in calcium release. Loss of phospholipase Cβ3 results in activation of stat5 and possibly reactive oxygen in the face of unopposed phospholipase Cγ, resulting in proliferation and increased vascular permeability. Structure of PIP2 from Wikipedia.10
Conflict-of-interest disclosure: J.L.A. is an inventor on triphenylmethane and fulvene technology mentioned in the commentary. ■