Abstract
As pediatric Hodgkin lymphoma (HL) survival rates approach > 95%, treatment decisions are increasingly based on minimizing late effects. Using a model-based approach, we explored whether the addition of radiotherapy contributes to improved overall long-term survival. We developed a state-transition model to simulate the lifetime HL clinical course, and we compared 2 treatment strategies: chemotherapy alone (CT) and chemoradiotherapy (CRT). Data on HL relapse, late recurrence, and excess second cancer and cardiac late-effects mortality were estimated from the published literature and databases. Outcomes included conditional life expectancy, cause-specific mortality, and proportion alive at age 50. For a hypothetical cohort of HL patients (diagnosis age 15), conditional life expectancy was 57.2 years with CT compared with 56.4 years with CRT. Estimated lifetime HL mortality risk was 3.6% with CT versus 2.2% with CRT. In contrast, combined risk of excess late-effects mortality was lower for CT (1.8% vs 7.4% with CRT). Among those alive at age 50, only 9.2% of those initially treated with CT were at risk for radiation-related late effects (100% for CRT). Initial treatment with CT may be associated with longer average per-person life expectancy. These results support the need for careful consideration of the risk-benefit profile of radiation as frontline therapy in pediatric patients.
Introduction
Hodgkin lymphoma (HL) accounts for ∼ 9% of all childhood cancers.1 Five-year survival rates with modern therapies are now approaching > 95%.2 The risks of late effects associated with radiation and chemotherapy, including second cancers and cardiac deaths, have become more widely recognized, and treatment decisions are increasingly based on minimizing late-effects risk and late mortality. Although radiation is no longer standard of care for all low- and intermediate-risk adult patients, it continues to be used for pediatric patients.3
Clinical studies suggest that chemotherapy alone (CT) can achieve disease control in a large proportion of patients, with radiation therapy used only for the subset who fail initial treatment or require salvage therapy after relapse. Because therapy for refractory or relapsed lymphoma involves higher doses of radiation and chemotherapy, patients who relapse face elevated risks for both second cancer and cardiac late-effects mortality. In contrast, chemoradiotherapy (CRT), which includes chemotherapy and radiotherapy, exposes all newly diagnosed patients to radiation, but because of its lower relapse rate, leads to fewer patients exposed to salvage therapy risks. For both treatment strategies, the combined impact of these mortality risks on overall survival (OS) is unclear.
Nachman et al investigated how short-term outcomes varied between pediatric HL patients who achieved a complete response with chemotherapy and were randomized to either (1) low-dose radiation treatment or (2) no further treatment.4 The study found that the CRT was associated with higher 3-year event-free survival (EFS) rate compared with CT. Randomization was halted because of the higher relapse rate observed with CT, but results on those randomized suggest disease control may be achieved without radiation in 85%-90% of HL patients.5,6
Risk of late-effects mortality varies by treatment regimen. Data from the French-British cohort of 5-year survivors of childhood cancers suggest that the relative risk of late-effects mortality increases with cumulative radiation and anthracycline dose.7,8 The relative risk of death from second malignant neoplasm increased with radiation dosage (Ptrend < .001) and the relative risk of death from cardiac causes increased with both radiation (Ptrend < .01) and anthracycline dosage (Ptrend = .02). Analyses based on the North American Childhood Cancer Survivors Study also found radiation (P < .01) and anthracycline dose (P < .01) to increase second malignant neoplasm risk.9
Although more intensive HL treatment may lead to lower relapse rates, patients may face a higher risk of late-effects mortality. At the same time, less intensive treatment may compromise initial disease control. If late-effects mortality risks are considered, how do OS rates compare between CT and CRT? At what relapse rate could a less aggressive treatment provide equivalent long-term survival outcomes? Given the available data, how certain are these findings? Whereas clinical studies provide insight on short- and intermediate-term survival and retrospective cohorts collect important data on late-effects mortality risks, clinical trials on the combined effects of short- and long-term mortality risks on OS are unlikely given the needed follow-up from time of treatment to 15 to 20 years after treatment. By leveraging the best available clinical data on short-term and late-effects mortality risks, decision-analytic and disease simulation methods can provide important and timely insight on these clinically relevant questions, highlight where better data are needed, and identify those factors most likely to influence outcomes.10 As such, we used a model-based approach to explore the trade-offs between short- and long-term mortality risks on OS for pediatric HL patients.
Methods
Model structure
Overview.
We developed a state-transition Markov model that simulates the clinical course of HL. At the beginning of the simulation, a hypothetical cohort of 15-year-old persons diagnosed with favorable, early-stage HL enters the model. Each month persons face a risk of dying from relapse, late recurrence, late effects, or background mortality. Late effects include deaths from second cancers and cardiac causes, and risks are dependent on initial treatment received. Persons are followed throughout their lifetime. Outcomes include conditional life expectancy, cause-specific lifetime mortality, and probability of being alive and at risk for late effects at age 50. We conducted sensitivity analyses to assess how key parameters and assumptions might influence results, including a probabilistic sensitivity analysis using second-order Monte Carlo simulations to more fully account for uncertainty. The model was constructed using TreeAge ProSuite 2008 (TreeAge Software).
Strategies.
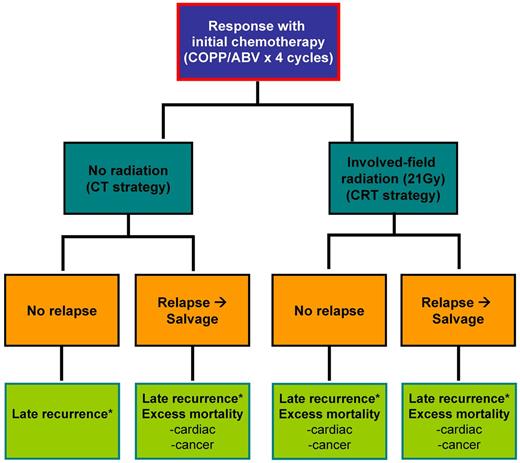
We focused on 2 general treatment strategies for HL patients who receive initial chemotherapy with 4 cycles of a standard HL regimen and achieve a complete response, as shown in Figure 1. These patients received either (1) no additional treatment (CT strategy) or (2) low-dose radiation (21 grays [Gy]; CRT strategy).4 All patients who relapsed received salvage therapy, which included additional chemotherapy, radiation therapy, and/or autologous stem cell transplantation. Based on cumulative treatment exposure, patients faced excess mortality risks for second cancer and cardiac events. The model is based on an assumption that patients receive a total dose of doxorubicin of 200 mg/m2 as a component of primary therapy. We did not include bleomycin-induced pulmonary mortality in our model as published studies on childhood cancer survivors suggest very minimal excess mortality risk.11,12
Model diagram. Patients diagnosed with HL who respond to initial chemotherapy receive either no additional treatment (CT strategy) or low-dose radiation (CRT strategy). Patients may relapse and receive salvage therapy. Patients then face risks for late recurrence and/or treatment-specific excess second cancer and cardiac mortality late effects. *Late-recurrence risk spans from 3 years (treatment completion) to 10 years since initial diagnosis.12 Cumulative dosage for COPP/ABV × 4: cyclophosphamide, 2400 mg/m2; vincristine, 5.6 mg/m; procarbazine, 2800 mg/m2; prednisone, 2240 mg/m2; doxorubicin, 140 mg/m2; bleomycin, 40 IU/m2; and vinblastine, 24 mg/m2.
Model diagram. Patients diagnosed with HL who respond to initial chemotherapy receive either no additional treatment (CT strategy) or low-dose radiation (CRT strategy). Patients may relapse and receive salvage therapy. Patients then face risks for late recurrence and/or treatment-specific excess second cancer and cardiac mortality late effects. *Late-recurrence risk spans from 3 years (treatment completion) to 10 years since initial diagnosis.12 Cumulative dosage for COPP/ABV × 4: cyclophosphamide, 2400 mg/m2; vincristine, 5.6 mg/m; procarbazine, 2800 mg/m2; prednisone, 2240 mg/m2; doxorubicin, 140 mg/m2; bleomycin, 40 IU/m2; and vinblastine, 24 mg/m2.
Mortality risks.
We estimated mortality risks from published studies and databases, as shown in Table 1.4,7,8,12-15 For the probability of relapse after initial treatment, we used event-free survival (EFS) estimates from a large randomized pediatric clinical trial comparing 3-year EFS for CT to CRT in patients with a complete response to upfront chemotherapy.4 For treatment-related late-effects, we based absolute excess risks on cause- and age-specific rates from U S life tables12,14 and relative risk estimates from the French-British cohort of childhood cancer survivors.7,8 Late HL recurrence mortality risk was based on published estimates from the North American Childhood Cancer Survivors Study.12 Survival after salvage therapy was based on a retrospective study of pediatric HL patients treated with additional chemotherapy, autologous stem cell transplantation, and radiation therapy.13
Model parameters: base case values and plausible ranges
| Variable . | Treatment dose . | Base case . | Plausible range . | Reference . |
|---|---|---|---|---|
| Treatment effectiveness (for patients with a complete response after initial chemotherapy), % | ||||
| 3-year EFS | 4 | |||
| CT | 87.0 | 84.8-89.2 | ||
| CRT | 92.0 | 90.1-93.9 | ||
| 5-year OS for salvage therapy | 74.2 | 58.7-84.6 | 13 | |
| Late HL recurrence, yearly risk, %* | ||||
| Mortality risk | 0.33 | 0.28-0.38 | 12 | |
| Late-effects mortality, relative risk†‡ | ||||
| CT | ||||
| Cardiac | 8 | |||
| Anthracycline, mg/m2 | < 239 | 1.0 | 0.1-5.5 | |
| CRT | ||||
| Cardiac | 8 | |||
| Radiation dose to heart, Gy | 5-14.9 | 12.5 | 1.4-116.1 | |
| Anthracycline, mg/m2 | < 239 | 1.0 | 0.1-5.5 | |
| Second cancer | 7 | |||
| Integral radiation dose, J§ | 41-149 | 2.1 | 1.12-3.8 | |
| Salvage | ||||
| Cardiac | 8 | |||
| Radiation dose to heart, Gy | ≥ 15 | 25.1 | 3.0-209.5 | |
| Anthracycline, mg/m2 | < 239 | 1.0 | 0.1-5.5 | |
| Second cancer | 7 | |||
| Integral radiation dose, J§ | ≥ 150 | 5.9 | 3.1-11.3 | |
| Variable . | Treatment dose . | Base case . | Plausible range . | Reference . |
|---|---|---|---|---|
| Treatment effectiveness (for patients with a complete response after initial chemotherapy), % | ||||
| 3-year EFS | 4 | |||
| CT | 87.0 | 84.8-89.2 | ||
| CRT | 92.0 | 90.1-93.9 | ||
| 5-year OS for salvage therapy | 74.2 | 58.7-84.6 | 13 | |
| Late HL recurrence, yearly risk, %* | ||||
| Mortality risk | 0.33 | 0.28-0.38 | 12 | |
| Late-effects mortality, relative risk†‡ | ||||
| CT | ||||
| Cardiac | 8 | |||
| Anthracycline, mg/m2 | < 239 | 1.0 | 0.1-5.5 | |
| CRT | ||||
| Cardiac | 8 | |||
| Radiation dose to heart, Gy | 5-14.9 | 12.5 | 1.4-116.1 | |
| Anthracycline, mg/m2 | < 239 | 1.0 | 0.1-5.5 | |
| Second cancer | 7 | |||
| Integral radiation dose, J§ | 41-149 | 2.1 | 1.12-3.8 | |
| Salvage | ||||
| Cardiac | 8 | |||
| Radiation dose to heart, Gy | ≥ 15 | 25.1 | 3.0-209.5 | |
| Anthracycline, mg/m2 | < 239 | 1.0 | 0.1-5.5 | |
| Second cancer | 7 | |||
| Integral radiation dose, J§ | ≥ 150 | 5.9 | 3.1-11.3 | |
Assumed persons face risk for late HL recurrence from 3 years (treatment completion) to 10 years since diagnosis.12
Assumed persons face risk for late-effects mortality beginning 5 years after initial HL diagnosis.
For cardiac and cancer-related late-effects, we assumed that background mortality rates were based on U S life tables.14 (1) persons faced excess mortality risk beginning 5 years after initial HL diagnosis; (2) based on data for the French-British cohort of childhood cancer survivors, absolute excess risks for late effects increased over time until age 25 for cardiac causes and age 35 for secondary or subsequent cancers and remained at these elevated levels thereafter (supplemental Methods and Figures, available on the Blood Web site; see the Supplemental Materials link at the top of the online article)12 ; and (3) excess mortality risks from radiation and anthracyclines were independent.7,8,16 For late HL recurrence, we assumed persons were at risk from completion of initial HL treatment to 10 years after initial diagnosis.12
Sensitivity analysis.
To portray the scope and nature of the uncertainties that surround our model outcomes, we conducted a wide range of sensitivity analyses. Univariate sensitivity analyses were performed to assess how changes in key model parameters affected base case estimates. We established a plausible range for each model variable using 95% confidence intervals and varied each one over its range while holding all other variables constant. In addition, to reflect patient time preferences for present versus future life-years gained, we explored how results varied under a range of discount rates. We also conducted 2-way sensitivity analyses on key model variables. To reflect the uncertainty in our estimates, we conducted a probabilistic sensitivity analysis using 100 000 second-order Monte Carlo simulations in which all parameter values were simultaneously varied (except for background mortality) using β distribution for initial treatment survival estimates (based on count data), and normal distributions for all other variables (based on 95% CI,17 assuming (1) a value of 0 for any negative numbers that arose from sampling for late HL recurrence risk, and (2) a value of 1 for any numbers less than 1, which would suggest a protective effect from radiation or anthracycline exposure, that arose from sampling for late-effects mortality relative risks).
Results
Conditional life expectancy
For patients treated with CT, the conditional life expectancy was 57.2 years, 0.8 years (1.5%) greater than for CRT (56.4 years; Table 2). For a cohort representative of the general population (who faced 0 risk of HL or late-effects mortality), the model estimated a conditional life expectancy of 60.9 years (< 1% discrepancy from the National Center for Health Statistics),18 which suggests a life-year loss of 3.7 years (6.0%) for HL patients treated with CT and 4.5 years (7.4%) with CRT.
Base case results for a cohort of 15-year-old HL patients
| Strategy . | Lifetime mortality probability, % . | Conditional LE . | Alive at age 50 . | Probability LE is higher* . | ||||
|---|---|---|---|---|---|---|---|---|
| HL . | Excess cancer . | Excess cardiac . | LE, yr . | Difference, yr (%) . | Proportion of cohort . | At risk for radiation-related late effects, % . | ||
| CT | 3.6 | 0.9 | 0.9 | 57.2 | 0.8 (1.5%) | 0.871 | 9.2 | 0.67 |
| CRT | 2.2 | 2.4 | 5.0 | 56.4 | 0.856 | 100 | 0.33 | |
| Strategy . | Lifetime mortality probability, % . | Conditional LE . | Alive at age 50 . | Probability LE is higher* . | ||||
|---|---|---|---|---|---|---|---|---|
| HL . | Excess cancer . | Excess cardiac . | LE, yr . | Difference, yr (%) . | Proportion of cohort . | At risk for radiation-related late effects, % . | ||
| CT | 3.6 | 0.9 | 0.9 | 57.2 | 0.8 (1.5%) | 0.871 | 9.2 | 0.67 |
| CRT | 2.2 | 2.4 | 5.0 | 56.4 | 0.856 | 100 | 0.33 | |
LE indicates life expectancy.
Based on 100 000 second-order Monte Carlo simulations using probabilistic sensitivity analysis.
Lifetime cause-specific mortality
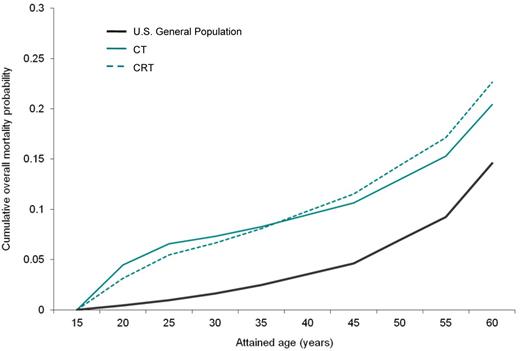
For a cohort of patients diagnosed with HL at age 15, CT, compared with CRT, had a higher lifetime mortality risk from HL (3.6% vs 2.2%), but lower combined risk of excess mortality from second cancers and cardiac events (1.8% vs 7.4%; Table 2). As shown in Figure 2, cumulative mortality was higher for CT until patients reached 36.6 years of age (ie, 21.6 years since initial diagnosis), after which CRT was associated with higher overall mortality.
Comparison of cumulative overall mortality. This figure shows the cumulative mortality probability for the base case. Solid green line represents CT; dotted green lines, CRT; and solid black line, U S general population.
Comparison of cumulative overall mortality. This figure shows the cumulative mortality probability for the base case. Solid green line represents CT; dotted green lines, CRT; and solid black line, U S general population.
Proportion of cohort alive and at risk at age 50
A greater proportion of the cohort was estimated to be alive at age 50 with CT than CRT (87.1% vs 85.6%). For CT, 91% of those alive at age 50 faced negligible excess cardiac or cancer-related mortality, and 9.0% faced a combined 9.3% excess cardiac and cancer mortality risk in their remaining lifetime because of successful salvage therapy. In contrast, for CRT, all persons who survived were at risk for late-effects mortality: 94% had a combined 3.5% risk of excess cardiac and second cancer mortality (2.4% and 1.1%, respectively), and the remaining 6% faced the combined excess mortality risk of 9.3% from salvage therapy after CRT.
Sensitivity analysis
We conducted sensitivity analyses to assess the robustness of results. We found that CT was the preferred strategy (ie, higher conditional life expectancy) unless its probability of short-term EFS decreased from 0.87 (base case) to 0.82, or the relative risk for CRT radiation-related cardiac mortality was reduced by more than 60% from 12.5 (base case) to 5.0. Even if the relative risk of CRT radiation-related second cancer mortality was 2-fold higher or salvage therapy was 100% effective, CT was still the preferred strategy. Results were insensitive to late-effects mortality risks from salvage therapy. If the relative risk for second cancer associated with anthracylines (< 240 mg/m2) increased to 2.1 (base case = 1.0),9 conditional life expectancy declined for both strategies (< 1%), but CT was still the preferred strategy compared with CRT (56.6 vs 55.8 years; difference = 0.8 years). If life-years gained were discounted at a rate of 3%,19 CT was still the preferred strategy (26.0 years vs 25.9 years with CRT), although the difference declined to 0.1% (base case = 1.4%). At discount rates exceeding 4.8%, CRT became the preferred strategy.
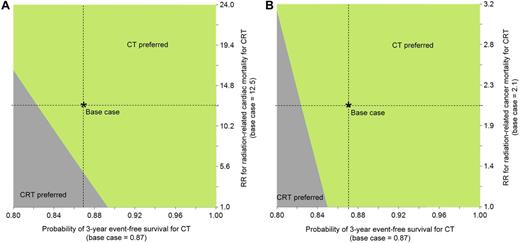
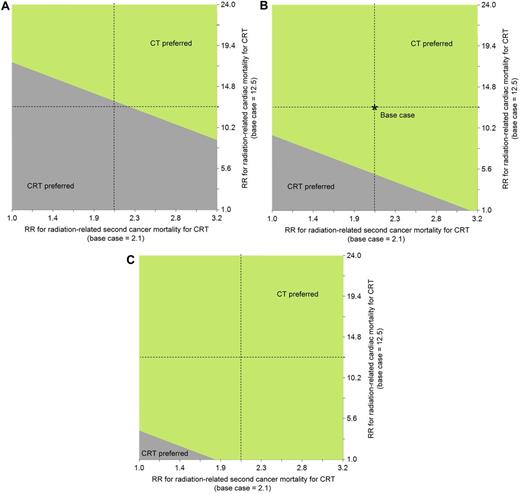
Figure 3 shows 2-way sensitivity analyses on short-term CT EFS and CRT radiation-related late-effects risks. If the relative risk for CRT cardiac mortality associated was 50% lower, CT was still preferable as long as its short-term EFS was higher than 0.86 (Figure 3A). If anthracycline exposure increased the CRT cardiac mortality relative risk associated with radiation by 2-fold, CT would also have a longer life expectancy (see below for related analyses on CT). Similarly, even if excess CRT cancer mortality risk was negligible, CT was still the preferred strategy unless its short-term EFS was less than 0.85 (Figure 3B). Figure 4 shows a 2-way sensitivity analysis on the CRT cardiac and second cancer mortality risks for various levels of short-term CT EFS. These figures suggest that at a short-term EFS associated with CT equal to 0.90, CT is the preferred strategy unless CRT late-effects mortality risks are approximately 50% lower (for second cancers) or almost negligible (for cardiac).
Two-way sensitivity analysis on short-term CT EFS and CRT risk for radiation-related late-effects mortality. These figures show 2-way sensitivity analyses on the probability of short-term CT EFS and CRT relative risk of radiation-related cardiac (A) and second cancer mortality (B). In both panels, the region where CT is preferred is indicated by the light green shaded area, and for CRT, the dark gray shaded area.
Two-way sensitivity analysis on short-term CT EFS and CRT risk for radiation-related late-effects mortality. These figures show 2-way sensitivity analyses on the probability of short-term CT EFS and CRT relative risk of radiation-related cardiac (A) and second cancer mortality (B). In both panels, the region where CT is preferred is indicated by the light green shaded area, and for CRT, the dark gray shaded area.
Two-way sensitivity analysis on CRT risk for radiation-related cardiac and second cancer mortality by various levels of short-term CT EFS. These figures show 2-way sensitivity analysis on CRT relative risk for radiation-related cardiac and second cancer mortality for the following levels of short-term CT EFS: probability = 0.82 (A), 0.87 (base case; B), and 0.90 (C). In each panel, the region where CT is preferred is indicated by the light green shaded area, and for CRT, the dark gray shaded area.
Two-way sensitivity analysis on CRT risk for radiation-related cardiac and second cancer mortality by various levels of short-term CT EFS. These figures show 2-way sensitivity analysis on CRT relative risk for radiation-related cardiac and second cancer mortality for the following levels of short-term CT EFS: probability = 0.82 (A), 0.87 (base case; B), and 0.90 (C). In each panel, the region where CT is preferred is indicated by the light green shaded area, and for CRT, the dark gray shaded area.
In addition, we conducted a series of scenario analyses to reflect differences in treatment regimen and salvage therapy. If we assumed that CT consisted of 6 cycles (vs 4 cycles for the CRT strategy) and/or was associated with higher relative risk of anthracycline-related cardiac late-effects mortality (RR = 1.3 for 240-359 mg/m2 exposure) compared with CRT (RR = 1.0 for < 239 mg/m2 exposure), CT was still preferable (57.2 vs 56.4 years; 1.4% difference).8 If salvage therapy for CRT was associated with a 10% lower survival rate and 10% higher mortality risk from late effects, the difference in life expectancy between CT and CRT (57.2 vs 56.1 year) increased from 1.5% (base case) to 2.0%.
In our base case, because absolute excess risks were based on relative risk estimates, we conservatively assumed that absolute excess risks increased until age 25 for cardiac disease and age 35 for second cancers, and remained elevated at this level for the remainder of the cohort's lifetime. If absolute excess risks leveled off 5 years later (ie, at age 30 for cardiac causes and age 40 for second cancers), CT was still the preferable strategy, and the magnitude of difference increased to 2.5 years, or 4.4% (base case = 0.8 years, or 1.4%). The age at which cumulative mortality was higher with CRT was 32.8 years (base case = 36.6 years of age).
To more fully account for uncertainty surrounding treatment effectiveness and excess mortality risks, we conducted probabilistic sensitivity analysis. Among 100 000 Monte Carlo simulations, the probability of CT having a greater life expectancy compared with CRT was 0.67. Among the simulations in which CT was associated with greater life expectancy than CRT, the average gain in life expectancy was 2.4 years (95% CI, 0.1-7.0 years).
Discussion
Motivated to minimize the risk of radiation-induced late-effects, in recent years, treatment for adult HL has focused primarily on chemotherapy-based protocols for initial therapy. Among pediatric patients, however, the change in approach has been more controversial largely because of concerns regarding higher relapse rates in children treated with chemotherapy only. Radiation in combination with chemotherapy continues to be standard practice, with a focus on minimizing exposure to chemotherapy agents known to have late toxicities, and reduction of dose and volume of radiation. Based on data on both short- and long-term mortality risks, our findings suggest that, among pediatric patients, initial treatment with only chemotherapy may lead to better patient outcomes long-term. Although the risk of mortality from HL is higher after CT compared with after CRT, the mortality risk from second cancer and cardiac late-effects is much lower when radiotherapy is avoided. As such, CRT may be associated with improved short-term EFS, but less aggressive initial treatment may lead to overall better patient outcomes and life expectancy.
Among adults treated for HL, less intensive initial treatments have been shown to have favorable short-term survival rates.20 Most recently, a clinical trial on limited-stage HL patients found that, at 12 years after treatment, ABVD therapy alone had higher OS than patients who received subtotal nodal radiation therapy, with or without 2 cycles of ABVD.21 The difference was the result of the number of deaths from non-HL causes, specifically the greater number of second cancers and cardiovascular events in the radiation-therapy group. Based on these findings, radiation therapy is no longer considered a component of the standard of care for initial treatment of adult limited-stage HL.3
Consistent with our findings, clinical studies suggest that, because effective salvage therapy is available, pediatric HL outcomes may be improved by considering overall, not just primary treatment,22 and long-term outcomes may be more important than low early relapse rate.23 Indeed, consideration of late effects may be even more important for pediatric patients given the probable higher risk of late effects from exposure during adolescent years, although more complicated because of the long periods of follow-up needed to adequately capture late mortality risks.6 As clinical trials that compare the outcome of CRT compared with CT cannot feasibly capture all mortality risks associated with late effects, our decision-analytic approach provides an informative tool for exploring the trade-offs between short- and long-term mortality risks.
Previous decision-analytic models have aimed to provide insight on HL treatment but have focused primarily on adult patients or specific late effects, such as secondary leukemia.24,25 A recent systematic review with meta-analysis of randomized controlled trials comparing CT with combined modality treatment in early-stage HL patients found that adding radiotherapy to chemotherapy improved tumor control and OS.26 Specifically, they found that CRT was associated with lower overall mortality risk until approximately 20 years after initial diagnosis, a finding that is consistent with our model estimates. The systematic review, however, was unable to consider the full impact of late effects in the comparison because of the limited observation times of the included trials included. Our study builds on their initial insights by taking into account a longer time horizon and suggesting that, if late-effects mortality risks are considered, CT may be the more attractive treatment for HL.
Although our findings suggest that CT may lead to move favorable survival outcomes, the life expectancy benefit appears to be small (0.8 years; or 1.5%). When we discounted life-years as a proxy for patient and family preferences for present versus future outcomes, we found that CT was still the preferred strategy unless the discount rate exceeded 4.8%, although the life expectancy benefit remained small. As such, patient preferences for short- and long-term mortality risks and the uncertainty surrounding them may be especially important factors to consider in treatment decisions. We also found that for CRT, the risk of dying from late effects was more than 3 times greater than from HL (7.4% vs 2.2%), emphasizing the importance of monitoring survivors' health. Prevention efforts, such as cancer and cardiac screening, may effectively reduce the risk of dying from these late effects. As the effectiveness of (and adherence to) these interventions become clearer, they will also be important factors for clinicians and patients to consider when weighing the trade-offs between short- and long-term mortality risks.
Our findings have some limitations. First, for short-term treatment effectiveness, we used published estimates from a randomized clinical trial on an older chemotherapy treatment regimen (cyclophosphamide, vincristine, procarbazine, prednisone [COPP]/doxorubicin, bleomycin, vinblastine [ABV]), which is no longer considered a standard regimen for low-risk HL. Newer chemotherapy regimens may include different anthracycline doses and avoid leukemogenic agents (such as procarbazine) but include others, such as etoposide; therefore, our estimate of second cancer mortality may not reflect that of modern regimens. We also did not account for any additive effects between chemotherapy and radiation on second cancer risk, which would lead to poorer OS for CRT (and those treated with CT who required salvage therapy). However, for these newer regimens, short-term mortality rates will continue to be an important determinant of the success of a CT strategy compared with CRT. Our model suggests that, even if the probability of short-term EFS is 0.95 for CRT, CT would still lead to a higher life expectancy as long as its short-term EFS is higher than 0.86.
We also did not include response-based treatment strategies in our analysis as our aim was to provide insight on the additional benefit of RT as part of initial treatment on OS. A recently published update of the randomized clinical trial on which our model is based reported outcomes in patients randomized to either involved-field radiotherapy or no further therapy.27 With the longer follow-up time (median time = 7.7 years), the study found that 10-year EFS was lower for patients treated with CT only (82.9% vs 91.2% for CRT), but that post-relapse survival rate was higher for those who experienced a relapse (82.5% vs 69.7% for CRT). As a result, there was no difference in OS between the 2 treatment groups for low-risk HL. In addition, no deaths from cardiac disease or secondary solid cancers were reported, probably a reflection of the fact that these deaths occur beyond the length of follow-up for this report. Using these updated trial results, our model suggests that CT leads to longer per-person life expectancy overall in accordance with our base case results. Indeed, the difference in life expectancy is greater (1.1 years, 1.9% using the updated data now available vs 0.8 years, 1.5% for base case) and based on probabilistic sensitivity analysis, the probability that CT is associated with greater life expectancy is even higher (0.74 vs 0.67 for base case).
The updated trial results, which suggest equivalent 10-year OS rates between CT and CRT, further elucidate the need for better determinants of which patients need to receive which therapies to obviate long-term toxicity and to maximize EFS and OS. Strategies that might affect choice of therapies, and therefore long-term outcomes, include identification of clinical features, which predict worse outcomes (eg, high-risk tumor histology or genetic features) and use of risk-adapted therapy, in which treatments associated with toxicities in specific patient groups are avoided (eg, sex-based therapies in which girls do not receive chest radiotherapy and thereby avoid late breast cancer mortality). In addition, response-based therapy, using early imaging response as a determinant of further therapy, now plays an important role in treatment choice. Initial results from the Children's Oncology Group Low Risk HL trial suggest that evaluation of fluorodeoxyglucose-positron emission tomography (FDG-PET) after even 1 cycle of chemotherapy may be predictive of EFS and identify groups of patients who require radiation or possibly more intensive chemotherapy approaches.28 The choice of further therapy for patients with inadequate responses after initial chemotherapy may be explored with our model, and as data with longer follow-up from response-based trials become available, our model can be adapted to estimate the potential benefit of response-based strategies on OS relative to CT, intensified CT, and CRT.
Another weakness of our study is that absolute excess risk estimates for late mortality were based on patients treated in Europe in previous decades. Although these data represent the best data on cause-specific late-effects mortality to date, late-effects risks for more recently treated patients may vary. In particular, radiation-associated late-effects risks may be influenced by changes in technique and dose. Modern therapies, such as involved field radiotherapy, may reduce the long-term secondary malignancy risk from radiation treatments and protect the heart and coronary arteries by reducing mean radiation dose to the heart.29 By limiting normal tissue exposure, these methods may be more important than total dose in terms of risk of long-term toxicities.30 As late-effects mortality data on survivors treated more recently will not be available for many years, our model provides important insight by leveraging the best available clinical data now, and suggests that if involved field radiation reduces CRT excess cardiac risk by 50% or eliminates CRT excess second cancer risk all together, CT would still be associated with more favorable OS. Furthermore, if 90% of patients can achieve short-term EFS with CT alone, excess risks for both second cancer and cardiac deaths would have to be significantly or entirely reduced for CRT to be the preferred strategy (Figure 4).
We also used broad treatment exposure groups for late-effects mortality risk estimates, which may not accurately estimate the late effects for CRT and life expectancy benefit for the CT strategy. For example, for excess cardiac risk, we assumed an intermediate level relative risk of 12.5 for initial CRT (mean radiation dose to heart = 5-14.9 Gy) and a high level relative risk of 25.1 for salvage therapy (mean dose ≥ 15.0 Gy), although both treatments probably included dosage closer to more than 30 Gy. We also used late-effects estimates from the French-British cohort, which used Gy to categorize radiation dose to the heart for excess cardiac risk and joules for integral radiation dose for excess second cancer risk,31,32 and probably reflect higher doses and larger fields than recommended low-dose regimens used today. Although we assumed that persons receiving CRT faced relative risks corresponding to the intermediate-level exposure group and salvage therapy patients faced high-level group risks, the exposure categories may not be directly comparable. We found, however, that when we used corresponding relative risk estimates in Gy for excess second cancer risk from a recent North American Childhood Cancer Survivors Study analysis on HL patients (RR = 7.4 for ≥ 30 Gy for CRT and salvage),9 results were similar and CT remained the preferred strategy with a life expectancy benefit of 3.1 years, or 5.5%. As such, our estimates likely provide conservative estimates of the impact of radiation late effects for the CRT treatment subgroup and the comparative benefit associated with CT.
We also focused only on cancer and cardiac late-effects, for which treatment-specific excess mortality has been observed. HL patients may face other risks associated with increased mortality; inclusion of other chemotherapy-associated mortality risks (eg, pulmonary disease) might influence the model, in particular, if there is a difference in risk in the 2 treatment strategies. Although childhood cancer survivor studies suggest that bleomycin does not increase the risk of dying,11,12 studies among adult HL patients have shown lower survival rates among those treated with the drug.33 As any excess mortality risks associated with bleomycin would affect OS for both CT and CRT (and CRT to a greater extent given the combined effects with radiation), our exclusion of any bleomycin-related mortality risks biases our results against CT and further strengthens our finding that CT leads to more favorable OS. As data on other toxicity risks become available, our model can be revised and updated to more comprehensively reflect the array of risks.
Our results also do not take into account treatment-related morbidity, such as nonfatal cancers and acute or chronic heart disease. Similarly, the model does not account for the impact of the 2 different strategies on medical care for HL patients. For example, female pediatric HL survivors who received chest radiation are recommended to undergo radiographic breast cancer screening beginning in their 20s, cancer screening that is not indicated until 20 years later in nonirradiated women. Similarly, HL patients, no matter how they were treated, may develop common diseases, such as breast cancer or coronary artery disease as they age; their initial treatment during childhood may influence their subsequent treatment options, their responses to therapy, or subsequent quality-of-life after treatment.
Finally, we focused our model on localized HL, largely because of the available data, which characterized survival outcomes after initial therapy with CT or CRT. However, using our model, we explored the implications for intermediate- and high-risk HL patients using short-term survival rates from Nachman et al.4 Similar to low-risk patients, CT was the preferred strategy for intermediate-risk patients (life expectancy benefit = 10-11 months). In contrast, for high-risk patients, CT was also associated with higher life expectancy, but the benefit was much smaller (< 1 month) and more sensitive to survival after salvage therapy. If the 5-year OS rate for salvage therapy was less than 70% (base case = 74%), CRT was the preferred strategy. As salvage therapy is less predictable for high-risk patients,34 radiotherapy is likely an important component of initial therapy for these patients for both short- and long-term outcomes.
For early-stage, favorable pediatric HL, model-based analyses relying on currently available data suggest that initial treatment with CT may be associated with longer average per-person life expectancy, reflecting the substantial long-term mortality risks associated with radiation therapy. Although caution is warranted in generalizing model-based results to individual patient clinical decision-making, our analysis supports the need for careful consideration and serious deliberation about the risk-benefit profile of radiation as frontline therapy in pediatric patients. We emphasize that model-based decision analyses, in addition to illuminating where better data would be most valuable, can be used to accommodate new information as it becomes available. Accordingly, additional data on late-effects mortality risks should be a priority for future clinical studies, permitting iterative reassessment of this analysis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (K07-CA143044, J.M.Y.).
National Institutes of Health
Authorship
Contribution: J.M.Y. and L.D. designed the study, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer M. Yeh, Center for Health Decision Science, Harvard School of Public Health, 718 Huntington Ave, 2nd Floor; Boston, MA 02115; e-mail: jyeh@hsph.harvard.edu.