Abstract
Dendritic cell (DC) migration via lymphatic vessels to draining lymph nodes (dLNs) is crucial for the initiation of adaptive immunity. We imaged this process by intravital microscopy (IVM) in the ear skin of transgenic mice bearing red-fluorescent vasculature and yellow-fluorescent DCs. DCs within lymphatic capillaries were rarely transported by flow, but actively migrated within lymphatics and were significantly faster than in the interstitium. Pharmacologic blockade of the Rho-associated protein kinase (ROCK), which mediates nuclear contraction and de-adhesion from integrin ligands, significantly reduced DC migration from skin to dLNs in steady-state. IVM revealed that ROCK blockade strongly reduced the velocity of interstitial DC migration, but only marginally affected intralymphatic DC migration. By contrast, during tissue inflammation, ROCK blockade profoundly decreased both interstitial and intralymphatic DC migration. Inhibition of intralymphatic migration was paralleled by a strong up-regulation of ICAM-1 in lymphatic endothelium, suggesting that during inflammation ROCK mediates de-adhesion of DC-expressed integrins from lymphatic-expressed ICAM-1. Flow chamber assays confirmed an involvement of lymphatic-expressed ICAM-1 and DC-expressed ROCK in DC crawling on lymphatic endothelium. Overall, our findings further define the role of ROCK in DC migration to dLNs and reveal a differential requirement for ROCK in intralymphatic DC crawling during steady-state and inflammation.
Introduction
Dendritic cells (DCs) are important in the initiation of adaptive immune responses. In peripheral tissues, such as the skin, DCs take up antigen, mature, and migrate via lymphatic vessels (LVs) to draining lymph nodes (dLNs), where they present antigen to resting T cells. Although this migration pattern has been known for more than 20 years,1 DC migration into LVs is only now starting to be unraveled at the cellular level using live imaging technologies.2-4 Before transmigrating across the lymphatic endothelium, DCs first need to squeeze through preformed, narrow pores present in the lymphatic basement membrane (BM).3 DC entry into LVs is thought to occur at the level of primary lymphatic capillaries, which feature discontinuous cell junctions with “button”-like accumulations of cell adhesion molecules.5 At the sites of such buttons, lymphatic endothelial cells (LECs) partially overlap and generate open flaps, through which leukocytes enter into lymphatics.3,5 The most prominent mediator of DC migration into afferent lymphatics is CCL21, a chemokine constitutively expressed in LVs.6,7 More recently, also other LEC-expressed molecules that mediate DC migration via LVs to dLNs have been identified.8-11 Notably, ICAM-1 and VCAM-1, which are up-regulated in LVs during inflammation,8,12 have been implicated in this process.8 Intriguingly, experiments performed with pan-integrin knockout DCs revealed that DC migration to dLNs in steady-state was integrin-independent.2 This apparent discrepancy could suggest that inflammation modulates the requirement for integrins and their ligands in DC migration, but this has not been conclusively addressed to date.
Until recently, it was commonly assumed that DCs that have transmigrated into the LV lumen are passively transported by the lymph flow.13 This assumption was supported by intravital microscopy (IVM) performed in rat mesentery lymphatics, where freely flowing lymphocytes are rapidly propagated in a pulsatile fashion.14 Lymph flow in lymphatic capillaries of peripheral tissues is difficult to measure and values ranging from 1 to 30 μm/s have been reported.15,16 Notably, these rates are considerably lower than the average lymph flow in large collecting LVs,14 making it questionable whether hydrodynamic forces present in the small lymphatic capillaries would be sufficiently strong to transport cells by flow. In fact, a recent IVM study revealed that DCs actively migrated within small lymphatic capillaries and were only propagated by lymph after they had reached larger collecting vessels.4 This suggests that leukocyte migration into and within LVs might involve similar adhesive interactions as leukocyte migration within and out of blood vessels (BVs). However, this possibility has been poorly explored and no molecules involved in active intralymphatic leukocyte migration have been identified so far.
Amoeboid cell movement is driven by 2 principal forces, namely actin polymerization and cellular extension occurring at the leading edge, and actomyosin-mediated cellular contraction and de-adhesion, which take place at the cell's rear.17,18 An important mediator of the latter 2 processes is the Rho-associated protein kinase (ROCK). ROCK controls the activity of nonmuscle myosin II, thereby promoting the reorganization and contraction of cellular actomyosin filaments.19 ROCK was shown to support cell movement by mediating nuclear contraction, which is required for migration through dense 3-D meshworks,2 and by mediating cellular de-adhesion from integrin ligands during leukocyte crawling on 2-D surfaces or on ICAM-1 expressed by blood vascular endothelium.20-22 However, the involvement of ROCK in DC migration in vivo has not been investigated to date.
In this study, we made use of a new transgenic mouse model with red-fluorescent vasculature to further investigate the role of ROCK in interstitial DC migration and migration into and within lymphatic capillaries. Performing IVM in the mouse ear skin we found that DC migration in the interstitium was ROCK-dependent. Interestingly, ROCK only played a minor role in intralymphatic DC migration at steady-state, whereas during tissue inflammation this process was highly ROCK-dependent. Further mechanistic analysis suggested that ROCK supports intralymphatic crawling during inflammation by mediating de-adhesion from inflammation-induced ICAM-1 on lymphatic endothelium. Overall, our findings indicate that inflammation alters the requirement for adhesion molecules in DC migration on lymphatic endothelium and represent the first description of molecules, which are involved in this process.
Methods
Mice
Wild-type (WT) C57BL/6 mice were purchased from Charles River laboratories or from Janvier. CCR7−/−23 and vascular endothelial (VE)–cadherin-Cre mice24 were from Jackson Laboratories. CD11c-YFP mice25 were kindly provided by Michel Nussenzweig (Rockefeller Institute, NY). VE-cadherin-Cre × RFP were generated by crossing Rosa26-loxP-stop-loxP-tdRFP mice26 with VE-cadherin-Cre mice. All experiments were approved by the Cantonal Veterinary Office Zurich.
Generation of BM chimeras
Four- to 6-week-old VE-cadherin-Cre × RFP mice were lethally irradiated (950 rad) and reconstituted with 5 × 106 BM cells isolated from CD11c-YFP mice. Chimeric mice were used at least 6 weeks after irradiation.
CHS-induced ear skin inflammation
A contact hypersensitivity (CHS) response toward oxazolone was induced as described.12 Briefly, mice were anesthetized by intraperitoneal administration of medetomidine (1 mg/kg) and ketamine (75 mg/kg) and sensitized by topical application of 2% oxazolone (4-ethoxymethylene-2-phenyl-2-oxazoline-5-1; Sigma-Aldrich) in acetone/olive oil (4:1 vol/vol) on the shaved abdomen (50 μL) and on each paw (5 μL). Five days later, 10 μL of a 1% oxazolone solution was applied topically to each side of the ears (challenge phase). Experiments were performed 24 to 48 hours after CHS challenge.
FACS analysis of ear single cell suspensions
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Ear whole-mount immunofluorescence
Mice were killed and ears harvested after hair removal with Veet depilation cream. Ears were split into 2 halves along the cartilage and blocked at room temperature for 30 minutes in 12% bovine serum albumin (BSA)/phosphate-buffered saline (PBS). Subsequently, ears were incubated for 1 hour at room temperature with either rat anti–mouse LYVE 1 (clone ALY727 ), rabbit anti–mouse LYVE-1 (AngioBio), hamster anti–mouse podoplanin (clone 8.1.112 ), rat anti–mouse MECA-32 (BD Bioscience), rabbit anti–mouse laminin (Sigma-Aldrich), or with corresponding isotype controls. Ears were washed with PBS/0.1% Tween-20 and incubated for at least 30 minutes with Alexa-conjugated secondary antibodies (Invitrogen) in presence of Hoechst 33342 (Invitrogen). Ears were washed with PBS and mounted using Vectashield (Vector Laboratories).
Image acquisition
Ear whole mounts were analyzed on a Zeiss LSM 710-FCS confocal microscope (Carl Zeiss AG). Images were acquired using Zeiss ZEN 2010 software (Carl Zeiss AG). Imaris Version 7.2.3 software (Bitplane AG) was used for offline image processing. In some cases γ-corrections were applied to better visualize LV morphology (stated in the legends for Figures 1,Figure 2–3).
Generation of BM-derived DCs
DCs were generated as previously described.28 A detailed description is provided in supplemental Methods.
Generation of splenic DCs
See supplemental Methods.
DC transfer and IVM in the ear
CD11c-YFP DCs were adjusted to 50 × 106/mL and transferred into an insulin syringe. For DC injection, mice were briefly anesthetized with isoflurane (Attane; Minrad), and cells in a total of 10 μL were injected into 2 to 3 regions in the ventral side of the mouse ear pinna. Four to 6 hours later, mice were anesthetized with medetomidine (1 mg/kg) and ketamine (75 mg/kg) and the ears depilated with Veet cream. Mice were transferred to a custom-made microscopy stage, lying on their back with the dorsal side of the ear facing the coverslip of the stage. Ears were moisturized with PBS and immobilized by carefully placing another coverslip on top. For imaging, mice were placed into a 37°C incubator chamber installed on the microscope platform. Imaging was performed on a Zeiss LSM 710 inverted confocal microscope (Carl Zeiss AG). All time-lapse videos were acquired using a 20 × 0.8 NA Plan Apochromat objective. An Argon laser (488 nm, for yellow fluorescent protein [YFP] excitation) and a solid-state laser (561 nm, for tandem dimer red fluorescent protein [tdRFP] excitation) were used for image acquisition. When tracking intralymphatic DC migration, a high-resolution image (1024 × 1024 pixel, signal averaging) was first acquired at the beginning of the experiment to prove intralymphatic localization of DCs. Subsequent image stacks were acquired with lower resolution in the red channel (512 × 512 pixel; with higher z-distance of images, no signal averaging, less laser power) to avoid photo damage and to allow for shorter stack intervals. z-dimensions of acquired image stacks typically ranged between 30 to 50 μm, and featured 20 to 30 slices with an overlap of ideally 50% (Nyquist Sampling). The individual slice depth typically was 3 to 4 μm, the acquired x/y-dimensions 707 × 707 μm.
To study the effect of Y27632 on DC motility, 2 consecutive, 60-minute time-lapse videos were acquired (30-second stack intervals) in 2 regions of interest. Subsequently, mice were treated by intraperitoneal injection of 10 mg/kg Y27632 (Sigma-Aldrich) or of PBS vehicle control. Mice were kept under anesthesia at 37°C for 3 hours. Subsequently, 2 consecutive 60-minute time-lapse videos were recorded in the 2 previously imaged regions.
Analysis of cell dynamics
Imaris Version 7.2.3 software (Bitplane) was used to analyze cell velocity, directionality and axis dimensions. Cell tracking was performed using automatic surface detection followed by manual cell tracking. Cells were included into statistical analysis if they displayed a motility > 1 μm/min and were trackable over at least 5 time points. In some experiments, the maximal cell length and width was measured using IMARIS.
FITC painting
See supplemental Methods.
Flow chamber experiments
See supplemental Methods.
Statistical analysis
Fluorescein isothiocyanate (FITC) painting data were analyzed using the Student t test (unpaired, 2-tailed) and are presented as mean ± SEM. Data from IVM or flow chamber experiments (velocities, axis-ratios) were analyzed using the Mann-Whitney test. Significant outliers, based on Grubbs test, were excluded. All experiments were repeated at least 2 times. Statistical analysis was performed with Prism 5 (GraphPad).
Results
Genetic mouse model to image LVs in vivo
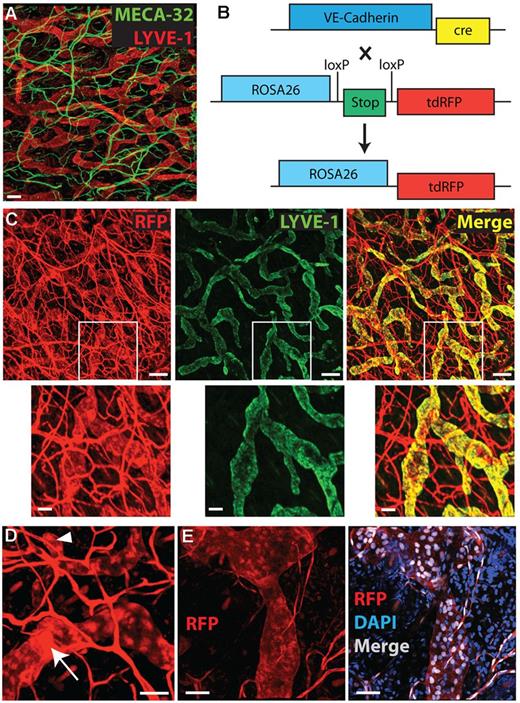
When studying the vasculature of the mouse ear skin by immunofluorescent analysis of whole mounts we noticed that BVs and LVs could easily be distinguished based on morphologic differences (Figure 1A). Most LVs, in particular lymphatic capillaries, were much wider than BVs. Furthermore, lymphatic capillaries could often be recognized by their blind beginnings, whereas lymphatic precollectors and the deeper lying collectors featured characteristic valve structures. We therefore reasoned that it should be possible to distinguish both vessel types in transgenic mice expressing 1 single fluorophore under the control of a pan-endothelial gene promoter, such as the VE-cadherin promoter.24 Therefore, we bred VE-cadherin-Cre mice24 with Rosa26-loxP-stop-loxP-tdRFP mice26 (Figure 1B). On Cre-activation, the latter mice express a genetically modified version of red fluorescent protein (RFP) under the ubiquitously active Rosa26 promoter.26,29,30 Analysis of the offspring, so-called VE-cadherin-Cre × RFP mice, revealed that the fluorescent signal in the vasculature was readily detectable in ear whole-mounts (Figure 1C). Costaining with LYVE-1 confirmed that BVs and LVs could be distinguished based on morphologic differences. Notably, virtually all LYVE-1–positive lymphatics also expressed RFP (Figure 1C). Blind beginnings of lymphatic capillaries and valve structures in precollecting and collecting LVs were readily detectable (Figure 1D). Interestingly, besides cytosolic expression, bright RFP expression was detected in cell nuclei of both blood vascular endothelial cells (BECs) and LECs (Figure 1E). Furthermore, some ear-resident leukocytes were RFP-positive (5%-12% of cells from different subsets, data not shown). Interestingly, further analysis revealed, that lymphatic collectors present in the deep dermis were RFP negative. By contrast, lymphatic capillaries present in the superficial dermis (ie, the presumed sites of leukocyte entry into lymphatics) were consistently RFP positive (supplemental Figure 1).
Analysis of the vasculature in WT and in VE-cadherin-Cre × RFP mice. (A) Immunofluorescent staining of blood (MECA-32, green) and lymphatic (LYVE-1, red) vessels in ear skin whole mount. Scale bar: 100 μm. (B) Mice with fluorescent BVs and LVs were generated by breeding VE-cadherin-Cre mice with Rosa26-loxP-stop-loxP-tdRFP mice. Cre-mediated deletion of the floxed stop-site induced transcription of tdRFP in VE-cadherin–expressing cells. (C-E) Analysis of ear whole mounts from the offspring, so-called VE-cadherin-Cre × RFP mice. (C) Co-staining for LYVE-1 (green), confirming RFP expression in both BVs and LVs. First row: Scale bar: 150 μm. Second row: high magnifications of white inserts. Scale bar: 50 μm. (D) High magnification view of an RFP-positive LV in an ear whole mount reveals a valve structure, as indicated by a white arrow. The large arrowhead shows a typical blind beginning of an initial LV. Scale bar: 50 μm. (E) RFP is expressed in the cytosol and in the nucleus, as evidenced by costaining for DAPI. Scale bar: 50 μm. Gamma corrections were applied to (A-C; only red channel) and (D-E) to enhance visibility of LVs.
Analysis of the vasculature in WT and in VE-cadherin-Cre × RFP mice. (A) Immunofluorescent staining of blood (MECA-32, green) and lymphatic (LYVE-1, red) vessels in ear skin whole mount. Scale bar: 100 μm. (B) Mice with fluorescent BVs and LVs were generated by breeding VE-cadherin-Cre mice with Rosa26-loxP-stop-loxP-tdRFP mice. Cre-mediated deletion of the floxed stop-site induced transcription of tdRFP in VE-cadherin–expressing cells. (C-E) Analysis of ear whole mounts from the offspring, so-called VE-cadherin-Cre × RFP mice. (C) Co-staining for LYVE-1 (green), confirming RFP expression in both BVs and LVs. First row: Scale bar: 150 μm. Second row: high magnifications of white inserts. Scale bar: 50 μm. (D) High magnification view of an RFP-positive LV in an ear whole mount reveals a valve structure, as indicated by a white arrow. The large arrowhead shows a typical blind beginning of an initial LV. Scale bar: 50 μm. (E) RFP is expressed in the cytosol and in the nucleus, as evidenced by costaining for DAPI. Scale bar: 50 μm. Gamma corrections were applied to (A-C; only red channel) and (D-E) to enhance visibility of LVs.
LVs can be distinguished from blood vessels in vivo
Confocal IVM performed in the ear skin of anesthetized mice confirmed that it was possible to detect fluorescent BVs and LVs in the dermis of VE-cadherin-Cre × RFP mice in vivo (Figure 2A-B). Imaging was performed at a depth of up to 70 μm, corresponding to the epidermis and most of the dermis in an uninflamed mouse ear. As expected from fluorescence-activated cell sorter (FACS) analysis, also RFP-expressing leukocytes were readily detectable, particularly in the epidermis (Figure 2C). At a depth of 15 to 25 μm, mainly superficial BVs were visible (Figure 2D), whereas LVs were predominately found in deeper regions of the dermis (Figure 2E). On intravenous injection of FITC-dextran (2000 kDa) only presumed RFP+ BVs, but no presumed RFP+ LVs filled with FITC-dextran (Figure 2F). Taken together, these findings confirmed the suitability of VE-cadherin-Cre × RFP mice for IVM studies.
IVM of the ear vasculature in VE-cadherin-Cre × RFP mice. Intravital confocal imaging was performed in the dorsal ear skin of anesthetized VE-cadherin × RFP mice. (A-B) Image stack visualizing RFP-positive blood vessels (BV), LVs (LV) and leukocytes (Leu) in panel A the lower dermis; in panel B the upper and lower dermis (z-dimension: 39 μm). Scale bars: 100 μm. (C-E) Split view of 1 layer in panel C the epidermis, containing many RFP+ cells, which presumably were Langerhans cells (LC); (D) the upper dermis containing many BVs organized around hair follicles (HF); and (E) the lower dermis containing the highest density in LVs. Scale bars: 100 μm. The number in the top-right corner indicates the distance to the epidermis. (F) Intravenously injected FITC-dextran (2000 kDa) only colocalized with RFP+ structures that were morphologically identified as blood vessels. In contrast, presumed LVs did not take up FITC-dextran. Scale bars: 100 μm. Gamma corrections were applied to panels A through E and red channel of panel F to enhance visibility of LVs.
IVM of the ear vasculature in VE-cadherin-Cre × RFP mice. Intravital confocal imaging was performed in the dorsal ear skin of anesthetized VE-cadherin × RFP mice. (A-B) Image stack visualizing RFP-positive blood vessels (BV), LVs (LV) and leukocytes (Leu) in panel A the lower dermis; in panel B the upper and lower dermis (z-dimension: 39 μm). Scale bars: 100 μm. (C-E) Split view of 1 layer in panel C the epidermis, containing many RFP+ cells, which presumably were Langerhans cells (LC); (D) the upper dermis containing many BVs organized around hair follicles (HF); and (E) the lower dermis containing the highest density in LVs. Scale bars: 100 μm. The number in the top-right corner indicates the distance to the epidermis. (F) Intravenously injected FITC-dextran (2000 kDa) only colocalized with RFP+ structures that were morphologically identified as blood vessels. In contrast, presumed LVs did not take up FITC-dextran. Scale bars: 100 μm. Gamma corrections were applied to panels A through E and red channel of panel F to enhance visibility of LVs.
DCs actively migrate within LVs
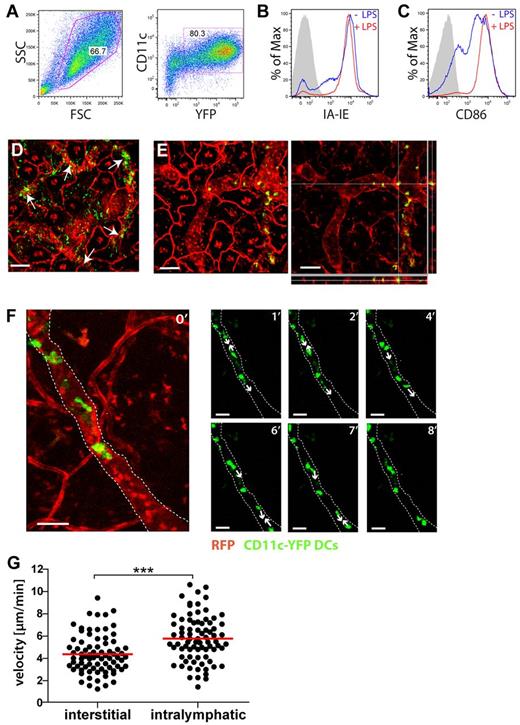
To image DC migration into and within LVs, lipopolysaccharide (LPS)-matured DCs, generated from the bone marrow (BM) of CD11c-YFP mice25 (Figure 3A-C), were adoptively transferred into the ear skin of VE-cadherin-Cre × RFP mice. Imaging was initiated 4 to 6 hours after transfer and performed in intact skin regions distant from the injection sites. At this time point, many YFP+ DCs were present in the interstitium or within LVs (Figure 3D-E). Notably, entry into LVs was CCR7-dependent, because adoptively transferred CCR7−/− DCs were much less frequently observed within LVs than WT DCs (supplemental Figure 2). Occasionally, also DCs that were in the process of migrating into LVs were imaged (supplemental Video 1). Analysis of videos revealed that most adoptively transferred DCs actively migrated within lymphatic capillaries (Figure 3F, supplemental Videos 2-3). DCs crawling within LVs were significantly faster than DCs migrating in the interstitial tissue (velocity of 5.7 μm/min vs 4.4 μm/min, respectively, P < .0001; Figure 3G). Although DC migration displayed an overall directionality within the vessel (supplemental Figure 3), we frequently observed intralymphatic DCs that changed their migratory path by 180° to migrate for some time in reverse direction of the expected lymph drainage (supplemental Video 4). Interestingly, experimentally elevating lymph flow by injection of PBS into the ear pinna did not seem to enhance the frequency of DC migration in the direction of presumed lymph flow (supplemental Figure 3D). A similar pattern of active intralymphatic migration was also observed when imaging adoptively transferred splenic DCs (supplemental Figure 4, supplemental Video 5). A second mode of motion, which was only rarely observed in the superficial lymphatic capillaries imaged in our setup, was rapid cell movement (≫ 100 μm/min). Such DCs had a round shape, indicating no interaction/crawling along the endothelium (supplemental Video 6, supplemental Figure 5).
Adoptively transferred BM-DCs colocalize and migrate within LVs. (A-C) Characterization of DCs generated from the BM of CD11c-YFP mice. (A) CD11c and YFP expression. (B-C) Overnight incubation in presence of LPS (0.1 μg/mL) induced further up-regulation of (B) I-A/I-E and of (C) CD86. (D-F) YFP+ DCs were injected into the ear skin and intravital imaging started after 4 to 6 hours. (D) Many DCs were found to colocalize with LVs, as indicated by white arrows. Scale bar: 100 μm. (E) Confocal analysis with z-axis projections confirming the intralymphatic location of a selected DC. Left: maximum intensity projection. Right: z-axis projection. Scale bar: 100 μm. (F) DCs actively migrate within LVs. Large picture on the left: confocal image stack showing green DCs within a red lymphatic vessel. Following pictures: sequential images showing elongated DCs that actively migrated within a lymphatic vessel. Numbers in the top-right corner indicate the minutes imaged. For simplicity reasons, only the green channel is shown. White lines outline the lymphatic vessel. White arrows indicate the direction of DC migration. Scale bars: 50 μm. (G) Quantification of the velocity of DC migration in the interstitium and within LVs. Each dot represents one tracked cell. Pooled data from 4 (interstitial) and 7 (intralymphatic) different experiments are shown (***P < .001). Gamma corrections were applied to panels D and E to enhance visibility of LVs.
Adoptively transferred BM-DCs colocalize and migrate within LVs. (A-C) Characterization of DCs generated from the BM of CD11c-YFP mice. (A) CD11c and YFP expression. (B-C) Overnight incubation in presence of LPS (0.1 μg/mL) induced further up-regulation of (B) I-A/I-E and of (C) CD86. (D-F) YFP+ DCs were injected into the ear skin and intravital imaging started after 4 to 6 hours. (D) Many DCs were found to colocalize with LVs, as indicated by white arrows. Scale bar: 100 μm. (E) Confocal analysis with z-axis projections confirming the intralymphatic location of a selected DC. Left: maximum intensity projection. Right: z-axis projection. Scale bar: 100 μm. (F) DCs actively migrate within LVs. Large picture on the left: confocal image stack showing green DCs within a red lymphatic vessel. Following pictures: sequential images showing elongated DCs that actively migrated within a lymphatic vessel. Numbers in the top-right corner indicate the minutes imaged. For simplicity reasons, only the green channel is shown. White lines outline the lymphatic vessel. White arrows indicate the direction of DC migration. Scale bars: 50 μm. (G) Quantification of the velocity of DC migration in the interstitium and within LVs. Each dot represents one tracked cell. Pooled data from 4 (interstitial) and 7 (intralymphatic) different experiments are shown (***P < .001). Gamma corrections were applied to panels D and E to enhance visibility of LVs.
To investigate whether this mode of active intralymphatic migration could also be observed in endogenous, skin resident DCs, BM chimeras were generated by reconstituting irradiated VE-cadherin-Cre × RFP mice with BM from CD11c-YFP mice. When imaging in uninflamed ear skin of BM chimeras, virtually no DCs were found to colocalize or migrate within LVs. By contrast, when imaging in LPS-inflamed ears of BM chimeras, many DCs were observed to migrate within LVs, displaying a similar pattern of active migration as observed in the adoptive transfer settings (supplemental Video 7).
ROCK blockade reduces DC migration to draining LNs
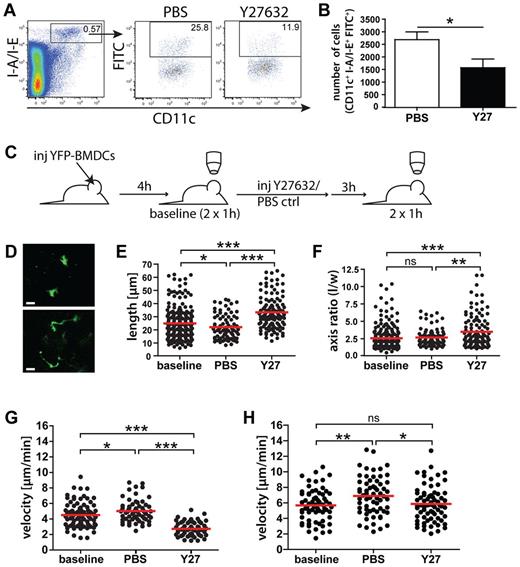
In agreement with a recent study,4 our data suggested that in addition to interstitial DC migration and transmigration into LVs, also DC movement within dermal lymphatic capillaries was dependent on active, amoeboid cell migration. The latter process generally requires Rac1/Rac2-mediated actin polymerization in the cellular front and concomitant actomyosin-mediated cellular contraction and de-adhesion occurring at the cellular rear.17,18 Indeed, simultaneous deletion of the small GTPases Rac1/Rac2 was shown to effectively impair DC migration to dLNs.31 By contrast, nothing is known about the importance of cellular contraction and de-adhesion in the migration of DCs from skin to dLNs. Because the latter 2 processes reportedly are mediated by ROCK,2 we decided to investigate the role of this kinase by blocking its activity with the well-described ROCK inhibitor Y27632.2,22 In a first analysis, FITC painting experiments were performed in the ears of Y27632-treated or PBS control-treated mice. In these experiments, significantly fewer FITC+ DCs were recovered from the ear-draining auricular LNs of Y27632-treated mice compared with the dLNs of control-treated mice (−41%, P = .028; Figure 4A-B).
Interstitial and intralymphatic DC migration is ROCK-dependent
To further explore the role of ROCK in interstitial and intralymphatic DC migration we studied these processes by IVM. For this, YFP+ DCs were adoptively transferred into the ears of VE-cadherin-Cre × RFP mice, and baseline motility of DCs in the interstitium and within lymphatics was recorded after 4 hours. Subsequently, mice were either treated with Y27632 or with PBS control and subjected to another round of imaging 3 hours later (Figure 4C). Intriguingly, treatment with Y27632 induced a striking shape change in interstitially migrating DCs, leading to significant cellular elongation and increased axis ratios (Figure 4D-F). This was probably because of the impairment of ROCK-mediated nuclear contraction, which caused the cells to get stuck with their nucleus in the dense network of the extracellular matrix (ECM).2 Accompanying these phenotypic changes, the average migratory speed of DCs within the interstitium was profoundly reduced by Y27632 treatment (−46% compared with PBS treatment; Figure 4G). Notably, the velocity of DC migration on PBS treatment was slightly increased compared with the velocity at baseline (Figure 4G). This could be because of heat or other manifestations of tissue damage, such as mild tissue inflammation induced by depilation of the ear, the cell injection process or prolonged imaging. Interestingly, Y27632 treatment also induced a significant, albeit very subtle reduction in the velocity of DC migration within LVs (−15% compared with PBS, P = .006; Figure 4H).
Interstitial and intralymphatic DC migration is ROCK-dependent. (A-B). FITC painting experiments were performed in the ears of WT mice treated with Y27632 (Y27) of with PBS vehicle control. Twelve hours after FITC application, mice were killed and the ear-draining auricular LNs harvested. (A) LN single cell suspensions were analyzed by FACS for the presence of I-A/I-E+CD11c+FITC+ cells. The number within each FACS plot represents the percentage of gated cells. (B) Y27632 treatment significantly reduced DC migration, as demonstrated by the quantification of total numbers of I-A/I-E+CD11c+FITC+ cells. Data from 1 of 5 similar experiments are shown. (C) Schematic representation of IVM experiments: YFP+ BM-DCs were injected into the ear skin of VE-cadherin-Cre × RFP mice, and baseline DC migration was recorded after 4 hours (2 × 1 hour). Two hours later, mice received intraperitoneal injections of Y27632 (10 mg/kg) or of PBS vehicle control. Imaging (2 × 1 hour) was initiated 3 hours after treatment. (D) Representative images of DCs present in the interstitium. Scale bar: 20 μm. (E) Y27632 treatment induced a significant elongation of interstitially migrating DCs. Quantification of the maximal DC length is shown. (F) Y27632 treatment induced a significant increase in the DC axis ratio, which was calculated by dividing the cellular length (l) by the cellular width (w). (G) Quantification of the effect of Y27632-treatment on the velocity of DC migration in the interstitium. (H) Quantification of the effect of Y27632-treatment on the velocity of intralymphatic DC migration. Dots represent individual cells. Pooled data from 6 (baseline) and 3 (PBS/Y27) different experiments are shown for panels E through G. Panel H shows pooled data from 5 (baseline), 3 (PBS), and 4 (Y27) different experiments. Baseline: before treatment; PBS: 3 to 4 hours after treatment with PBS; and Y27632: 3 to 4 hours after treatment with Y27632 (*P < .05; **P < .01; ***P < .001).
Interstitial and intralymphatic DC migration is ROCK-dependent. (A-B). FITC painting experiments were performed in the ears of WT mice treated with Y27632 (Y27) of with PBS vehicle control. Twelve hours after FITC application, mice were killed and the ear-draining auricular LNs harvested. (A) LN single cell suspensions were analyzed by FACS for the presence of I-A/I-E+CD11c+FITC+ cells. The number within each FACS plot represents the percentage of gated cells. (B) Y27632 treatment significantly reduced DC migration, as demonstrated by the quantification of total numbers of I-A/I-E+CD11c+FITC+ cells. Data from 1 of 5 similar experiments are shown. (C) Schematic representation of IVM experiments: YFP+ BM-DCs were injected into the ear skin of VE-cadherin-Cre × RFP mice, and baseline DC migration was recorded after 4 hours (2 × 1 hour). Two hours later, mice received intraperitoneal injections of Y27632 (10 mg/kg) or of PBS vehicle control. Imaging (2 × 1 hour) was initiated 3 hours after treatment. (D) Representative images of DCs present in the interstitium. Scale bar: 20 μm. (E) Y27632 treatment induced a significant elongation of interstitially migrating DCs. Quantification of the maximal DC length is shown. (F) Y27632 treatment induced a significant increase in the DC axis ratio, which was calculated by dividing the cellular length (l) by the cellular width (w). (G) Quantification of the effect of Y27632-treatment on the velocity of DC migration in the interstitium. (H) Quantification of the effect of Y27632-treatment on the velocity of intralymphatic DC migration. Dots represent individual cells. Pooled data from 6 (baseline) and 3 (PBS/Y27) different experiments are shown for panels E through G. Panel H shows pooled data from 5 (baseline), 3 (PBS), and 4 (Y27) different experiments. Baseline: before treatment; PBS: 3 to 4 hours after treatment with PBS; and Y27632: 3 to 4 hours after treatment with Y27632 (*P < .05; **P < .01; ***P < .001).
ROCK is more important for intralymphatic DC migration during inflammation than during steady-state conditions
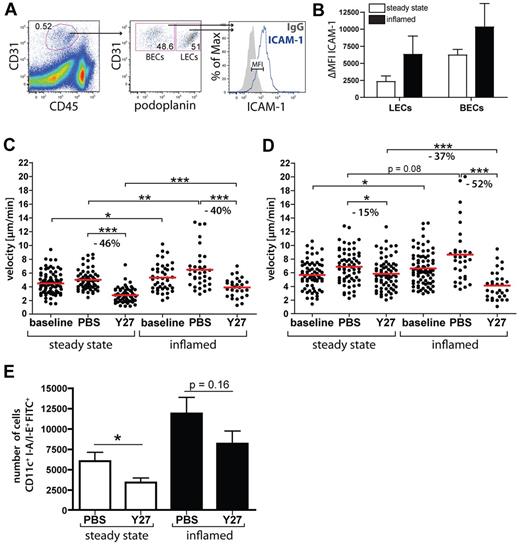
Several recent studies have shown that ROCK and nonmuscle myosin II are required for lymphocyte crawling on blood vascular endothelium, supposedly by mediating the de-adhesion of LFA-1 at the cellular uropod from endothelial cell–expressed ICAM-1,20-22 but no reports on a similar role for ROCK in DCs exist. ICAM-1 reportedly is expressed at very low levels in uninflamed LVs but becomes up-regulated in response to inflammation.8,12 Performing FACS analysis of ear single-cell suspensions (Figure 5A) we found that inflammation, induced by a CHS response to oxazolone, lead to an up-regulation of ICAM-1 in LECs, resulting in ICAM-1 expression levels similar to the ones found in uninflamed BECs (Figure 5B). To investigate whether inflammation-induced up-regulation of ICAM-1 in LECs would enhance the ROCK-dependence of intralymphatic DC migration, IVM was performed in CHS-inflamed ear skin. Similar to what we had observed in uninflamed conditions, ROCK blockade significantly reduced the speed of interstitial DC migration during inflammation (Figure 5C). Interestingly, interstitial as well as intralymphatic DC migration was slightly faster in inflamed compared with resting tissue (Figure 5C-D). In contrast to the only slight reduction observed under uninflamed conditions (−15%), Y27632 treatment profoundly reduced the speed of intralymphatic DC migration (−52%) in inflamed LVs (Figure 5D). Moreover, in presence of tissue inflammation, the migration velocity of Y27632-treated DCs was significantly reduced compared with the velocity of Y27632-treated DCs migrating in uninflamed LVs (−37%, P < .0001; Figure 5D). Thus, the contribution of ROCK to intralymphatic DC migration was more pronounced in inflamed compared with uninflamed LVs, supporting our hypothesis that ROCK mediates DC de-adhesion from LEC-expressed ICAM-1 during intralymphatic migration. Notably, ROCK blockade did not alter the expression levels of LFA-1 and Mac-1 in DCs (data not shown).
Inflammation enhances the ROCK dependence of intralymphatic DC migration. (A) Gating scheme used for the analysis of ICAM-1 expression in LECs and BECs: CHS-inflamed and control ears of WT mice were enzymatically digested, stained for CD45, CD31, and podoplanin and analyzed by FACS for ICAM-1 expression in LECs (CD45−CD31+podoplanin+) and BECs (CD45−CD31+podoplanin−). (B) FACS analysis revealed an inflammation-induced up-regulation of ICAM-1 in LECs and BECs. ΔMFIs are shown, defined as the difference in the median fluorescent intensity between ICAM-1 expression and the corresponding isotope control staining. Representative data from 1 of at least 3 similar experiments are shown. (C-D) YFP+ BM-DCs were injected into the control or CHS-inflamed ear skin of VE-cadherin-Cre × RFP mice. (C) Quantification of the effect of Y27632-treatment (Y27) on the speed of interstitial DC migration in resting versus CHS-inflamed ear skin. (D) Quantification of the effect of Y27632-treatment on the speed of intralymphatic DC migration in resting versus CHS-inflamed ear skin (same dosing scheme as in Figure 4C). Inflamed dataset is pooled from 9 (baseline), 4 (PBS), and 5 (Y27) different experiments. (E) FITC painting experiments were performed in the control of CHS-inflamed ears of mice treated with Y27632 of with PBS vehicle control. Y27632 treatment reduced DC migration, as demonstrated by the quantification of total numbers of I-A/I-E+CD11c+FITC+ cells. Data from 1 of 3 similar experiments are shown (*P < .05; **P < .01; ***P < .001).
Inflammation enhances the ROCK dependence of intralymphatic DC migration. (A) Gating scheme used for the analysis of ICAM-1 expression in LECs and BECs: CHS-inflamed and control ears of WT mice were enzymatically digested, stained for CD45, CD31, and podoplanin and analyzed by FACS for ICAM-1 expression in LECs (CD45−CD31+podoplanin+) and BECs (CD45−CD31+podoplanin−). (B) FACS analysis revealed an inflammation-induced up-regulation of ICAM-1 in LECs and BECs. ΔMFIs are shown, defined as the difference in the median fluorescent intensity between ICAM-1 expression and the corresponding isotope control staining. Representative data from 1 of at least 3 similar experiments are shown. (C-D) YFP+ BM-DCs were injected into the control or CHS-inflamed ear skin of VE-cadherin-Cre × RFP mice. (C) Quantification of the effect of Y27632-treatment (Y27) on the speed of interstitial DC migration in resting versus CHS-inflamed ear skin. (D) Quantification of the effect of Y27632-treatment on the speed of intralymphatic DC migration in resting versus CHS-inflamed ear skin (same dosing scheme as in Figure 4C). Inflamed dataset is pooled from 9 (baseline), 4 (PBS), and 5 (Y27) different experiments. (E) FITC painting experiments were performed in the control of CHS-inflamed ears of mice treated with Y27632 of with PBS vehicle control. Y27632 treatment reduced DC migration, as demonstrated by the quantification of total numbers of I-A/I-E+CD11c+FITC+ cells. Data from 1 of 3 similar experiments are shown (*P < .05; **P < .01; ***P < .001).
IVM performed in LPS-inflamed ears of BM chimeras revealed that interstitial and intralymphatic DC migration was similarly reduced on treatment with Y27632, indicating a similar requirement for ROCK in endogenous DCs (supplemental Figure 6). To investigate how ROCK blockade affected the overall process of DC migration from the skin to dLNs in the context of inflammation, we again performed FITC painting experiments. DC migration from CHS-inflamed ears to dLNs was significantly enhanced by inflammation, as previously reported.12 Also in the context of inflammation, ROCK blockade lead to a decrease in DC migration, but this effect did not reach statistical significance (Figure 5E).
Blockade of ICAM-1 or of ROCK reduces DC crawling velocity on LECs in vitro
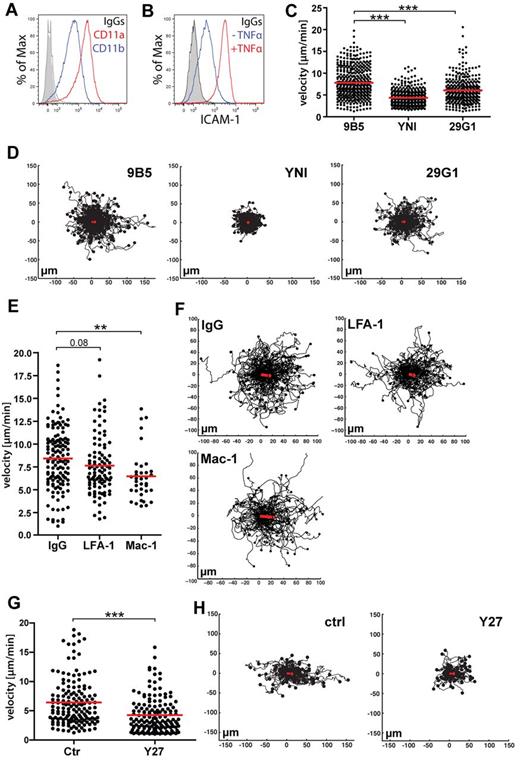
Our findings described in the previous paragraph suggested that up-regulation of inflammation-induced ICAM-1 in LECs could be functionally linked to the increased ROCK dependence observed for intralymphatic DC migration in the context of inflammation. FACS analysis confirmed that BM-DCs expressed high levels of CD11a and CD11b, which form part of the ICAM-1 ligands LFA-1 and Mac1 (Figure 6A). To further study the role of ROCK in DC crawling on lymphatic endothelium, in vitro flow chamber experiments were performed with BM-DCs and conditionally immortalized LECs (imLECs).12 Confluent imLEC monolayers were cultured overnight in presence of TNFα and IFNγ to mimic tissue inflammation and to up-regulate ICAM-1 expression (Figure 6B).12 To simulate physiologic conditions in primary lymphatic capillaries in inflamed tissues15,16 experiments were performed in presence of low flow. Under these conditions, DCs avidly crawled on imLECs (supplemental Video 8). Pre-treatment of LECs with 2 different ICAM-1 blocking antibodies significantly reduced the speed of DC migration, compared with pre-treatment with an unspecific control antibody (Figure 6C-D), thus confirming a role for ICAM-1 in DC crawling on lymphatic endothelium. Further experiments with blocking antibodies revealed that both LFA-1 and Mac-1 were involved in DC crawling on lymphatic endothelium (Figure 6E-F). Similarly to our IVM experiments, treatment of DCs with Y27632 resulted in a significantly slower speed of DC crawling on LECs (Figure 6G-H). Because DCs were pre-incubated with Y27633 and washed before the experiment, one can conclude from these findings that blockade of DC-expressed ROCK was sufficient to reduce DCs crawling on lymphatic endothelium.
In vitro DC migration on inflamed lymphatic endothelium is ICAM-1 and ROCK-dependent. (A) FACS analysis of CD11a and CD11b expression in LPS-matured BM-DCs. (B-F) Flow chamber experiments were performed on monolayers of TNFα/IFNγ-stimulated murine imLECs, in presence of low shear-stress conditions (0.15 dyne/cm2). (B) Overnight stimulation with TNFα/IFNγ lead to a strong up-regulation of ICAM-1 expression in LECs, as demonstrated by FACS. One of at least 5 independent experiments is shown. (C) Pretreatment of LECs with ICAM-1 blocking antibodies (clone YN1/1.7.4 and 29G1), significantly reduced the speed of DC migration on LECs in comparison to pretreatment with an unspecific control antibody (clone 9B5). (D) Track plots of DCs migrating on anti-ICAM1 and ctrl-treated LECs. The starting point of each track was set to the center point of the respective diagram. End points of tracks are indicated by dots. The red line indicates the overall migration directionality and represents the addition of the displacement vectors of all tracked cells. (E) Pretreatment of DCs with LFA-1 or Mac-1 blocking antibodies (clone FD441.8 and M1/70, respectively) reduced the speed of DC migration on LECs in comparison to pretreatment with an unspecific control antibody (clone A95-1). (F) Track plots of anti–LFA-1, anti–Mac-1, or control-treated DCs. (G) Pretreatment of DCs with Y27632 (Y27) significantly reduced the speed of DC migration on LECs. (H) Track plots of Y27632 and control-treated DCs migrating on LECs. Pooled data from 3 (Y27/ctrl/29G1/), 4 (9B5), 5 (YNI) and 6 (FD441.8/M1/70/A95-1) different experiments are shown in panels C through F (*P < .05; **P < .01; ***P < .001).
In vitro DC migration on inflamed lymphatic endothelium is ICAM-1 and ROCK-dependent. (A) FACS analysis of CD11a and CD11b expression in LPS-matured BM-DCs. (B-F) Flow chamber experiments were performed on monolayers of TNFα/IFNγ-stimulated murine imLECs, in presence of low shear-stress conditions (0.15 dyne/cm2). (B) Overnight stimulation with TNFα/IFNγ lead to a strong up-regulation of ICAM-1 expression in LECs, as demonstrated by FACS. One of at least 5 independent experiments is shown. (C) Pretreatment of LECs with ICAM-1 blocking antibodies (clone YN1/1.7.4 and 29G1), significantly reduced the speed of DC migration on LECs in comparison to pretreatment with an unspecific control antibody (clone 9B5). (D) Track plots of DCs migrating on anti-ICAM1 and ctrl-treated LECs. The starting point of each track was set to the center point of the respective diagram. End points of tracks are indicated by dots. The red line indicates the overall migration directionality and represents the addition of the displacement vectors of all tracked cells. (E) Pretreatment of DCs with LFA-1 or Mac-1 blocking antibodies (clone FD441.8 and M1/70, respectively) reduced the speed of DC migration on LECs in comparison to pretreatment with an unspecific control antibody (clone A95-1). (F) Track plots of anti–LFA-1, anti–Mac-1, or control-treated DCs. (G) Pretreatment of DCs with Y27632 (Y27) significantly reduced the speed of DC migration on LECs. (H) Track plots of Y27632 and control-treated DCs migrating on LECs. Pooled data from 3 (Y27/ctrl/29G1/), 4 (9B5), 5 (YNI) and 6 (FD441.8/M1/70/A95-1) different experiments are shown in panels C through F (*P < .05; **P < .01; ***P < .001).
Discussion
In this study, we made use of IVM and of transgenic mice with red-fluorescent vasculature to analyze the role of ROCK in intralymphatic DC migration and in the overall process of DC migration from skin to dLNs.
Gene-targeted mice expressing a Cre-recombinase or a fluorophore under an LEC-specific promoter have recently been generated,32-36 but only 2 IVM studies performed in such mice have been reported so far.35,36 Therefore, to date, LVs have mainly been visualized in IVM by in vivo labeling with intracutaneously injected fluorescent antibodies.4,37,38 However, antibody labeling is considered less optimal for IVM than endogenous expression of fluorophores; it is typically restricted to the injection site and downstream regions and could interfere with leukocyte-endothelium interactions or lead to unwanted immune activation. To bypass these potential problems, we bred mice expressing Cre-recombinase under the pan-endothelial VE-Cadherin promoter24 with RFP reporter mice.26 IVM experiments and confocal whole-mount analyses confirmed that it was possible to distinguish BVs and LVs in the ear skin of VE-cadherin-Cre × RFP mice based on morphologic differences. Furthermore, initial lymphatic capillaries, which are the main sites of leukocyte entry into LVs,3,5 uniformly were RFP+ in VE-cadherin-cre × RFP mice, confirming the suitability of this transgenic model for imaging DC migration into and within lymphatic capillaries.
In agreement with a recent study by Tal and colleagues, our IVM experiments revealed that adoptively transferred as well as endogenous, skin resident DCs actively migrated within superficial lymphatic capillaries.4 Although the imaging of YFP+ skin-resident DCs in BM chimeras represented a more physiologic approach, it proved difficult to use this setup for an in-depth mechanistic analysis of intralymphatic DC migration. Even on injection of LPS into the ear pinna, to induce DC maturation and migration, the number of DCs found within a LV segment typically was low, making it difficult to quantitatively investigate intralymphatic DC migration. This is probably because of the much lower number and density of endogenous DCs in the ear skin, compared with high numbers of BM-DCs deposited at the injection site. Consequently, in the latter approach, the probability of finding many DCs in the same LV are much higher. As mentioned in “DCs actively migrate within LVs,” we only rarely observed intralymphatic DCs, which were round-shaped and were flushed through LVs by flow. This is probably explained by our confocal imaging setup, which did not allow us to image in deeper regions of the dermis, where collecting vessels are most abundant, in which DCs are mainly transported by flow.4
Our IVM experiments revealed that interstitial DC migration was significantly reduced on ROCK blockade. These in vivo findings for the first time confirm previous observations made in vitro with DCs migrating in dense 3-D collagen matrices.2 The fact that DCs in the ears of Y27632-treated mice adopted a more elongated shape compared with DCs in control treated mice suggests that interstitial movement requires actomyosin-mediated contraction of the cell nucleus, to navigate through the dense network of the dermal ECM. Given that the composition and density of the ECM is tissue-specific,39 it is probable that also the requirement for ROCK-mediated nuclear contraction during interstitial DC migration varies in different tissues. Interestingly, in steady-state, inhibition of ROCK only marginally reduced the speed of DCs crawling within LVs, indicating that LVs provide a largely unconfined, 2-D–like environment for DC migration. However, initial capillaries often exhibit a rather flattened and collapsed phenotype with a compressed lumen in steady-state. Therefore, it is possible that some nuclear contraction and squeezing is still required for DC migration through collapsed lymphatic capillaries. In the context of tissue inflammation, which is accompanied by lymphatic vessel dilation and up-regulation of integrin ligands, such as ICAM-1, it is more probable that the role of ROCK is to mediate de-adhesion of DC-expressed integrins from their LEC-expressed ligands. Notably, ROCK inhibition did not lead to a complete block of interstitial or intralymphatic DC migration, indicating that besides ROCK, also other kinases might mediate nuclear contraction and integrin de-adhesion in DCs. However, although several kinases reportedly regulate nonmuscle myosin II activity, ROCK presently is the only one with a documented role in leukocyte de-adhesion.19
In vitro and in vivo studies have shown that ROCK is critical for leukocyte transmigration through blood vascular endothelium.22,40 Furthermore, it was recently reported that actomyosin-mediated nuclear contraction, induced by LEC-expressed semaphorin 3A, was important for DC entry into LVs.10 Given these findings and the fact that ROCK is important for DC migration through dense collagen meshworks in vitro,2 it is rather probable that ROCK is required for DC transmigration through the perilymphatic BM and subsequent entry into lymphatic capillaries. Interestingly, when imaging DCs in Y27632-treated mice we observed one DC that seemed to be arrested in the transmigration process (supplemental Video 9). However, not sufficient transmigration events (supplemental Videos 6 and 9) could be recorded to allow for a statistical analysis of this phenomenon.
Although our IVM studies showed that in inflammation both interstitial and intralymphatic DC migration were strongly ROCK-dependent, the inhibitory effect of Y27632 on DC migration from skin to dLNs was less strong in inflamed compared with steady-state conditions. It is possible that inflammation-induced changes in the lymphatic vessel architecture or the surrounding BM might render the transmigration process less dependent on actomyosin-mediated nuclear contraction. However, when analyzing the expression of laminin in the perilymphatic BM we could not detect any clear differences in the pre-existing pores, through which DCs reportedly enter into lymphatics.3 By contrast, we observed that the overall staining intensity for laminin around lymphatic initials was significantly reduced in inflamed compared with steady-state ear skin (supplemental Figure 7). This could indicate that the BM becomes thinner and less rigid in inflammation, what could render the transmigration process less ROCK-dependent. Interestingly, a similar inflammation-induced thinning of the BM was already reported for BVs.41
It was recently shown that under steady-state conditions, integrin-deficient DCs homed as efficiently to dLNs as WT DCs, suggesting that interactions between DC-expressed integrins and LEC-expressed integrin ligands were not important in the process of DC migration in the interstitium and into or within LVs.2 By contrast, an earlier study identified a role for inflammation-induced ICAM-1 or VCAM-1 in mediating DC migration from inflamed skin to dLNs.8 The new data from our work helps to solve this discrepancy, as they provide evidence that the requirement for adhesion molecules in DC migration varies between steady-state and inflammation. A probable candidate for such inflammation-dependent effects is ICAM-1, which becomes highly up-regulated in inflamed LECs but is only expressed at low levels in steady-state. Several studies have documented the role of ICAM-1 in leukocyte crawling on blood vascular endothelium in vivo42,43 and in vitro.44 In our flow-chamber assays, blockade of ICAM-1 or its DC-expressed integrin ligands significantly reduced the speed of DC crawling on activated lymphatic endothelium, suggesting a similar role for ICAM-1 in intralymphatic DC migration. Inflammation-induced ICAM-1 on LECs therefore may support the migration of DCs, by providing anchoring residues at the leading edge. At the same time, DCs require ROCK to mediate de-adhesion from LEC-expressed ICAM-1 at the uropod. It is possible that in addition to ICAM-1, other LEC-expressed integrin ligands also participate in this process. Notably, VCAM-1 is also induced in inflamed LECs in vivo,8,12 but its expression is lower compared with the one of ICAM-1 (data not shown).
What could be the biologic meaning of intralymphatic DC crawling? Measurements of lymphatic flow in lymphatic capillaries have indicated values in the order of a few μm/s.15,16 Notably, these velocities are much higher than the speed of active intralymphatic DC crawling measured by us and by others.4 If, on entry into lymphatic capillaries, DCs were exclusively transported by flow, their movement from the ear skin or footpad to the respective dLN (distance of < 2 cm) should, in theory, take a bit more than 1 hour (assuming that cells were traveling with a median lymph flow velocity of 4.7 μm/s15 ). However, studies performed in mice to evaluate the kinetics of DC migration in response to contact sensitization have revealed that it takes much longer before the first DCs are detected in skin dLNs, and maximal numbers of migrated DCs are only reached after 24 hours.45,46 Mobilization of dermal DCs from the interstitial space to the lymphatic vessel in response to contact sensitization was recently shown to occur within 1 hour of DC stimulation,38 and DC transmigration into lymphatic capillaries reportedly occurs within 2 hours (Tal et al,4 Sen et al,38 and supplemental Video 1). Thus, it is questionable whether the overall time needed for DC mobilization, transmigration, and passive intralymphatic transport by flow could explain the long time required for DC migration from skin to dLNs. Therefore, DC crawling within lymphatic capillaries might be a rate-limiting and essential step for mobilizing DCs from lymphatic capillaries to the downstream collectors, in which flow conditions are sufficiently strong to support passive transport.
Overall, our results provide first mechanistic insights into the molecules mediating DC crawling within lymphatic vessels. Our findings demonstrate that DC transport within lymphatic vessels is a more complex process than originally anticipated and probably regulated by similar adhesive mechanisms as the well-explored migration and extravasation of leukocytes out of blood vessels.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carlos Ochoa (ETH Zurich) and Mark Liebi (Theodor Kocher Institute, Bern) for excellent technical assistance and Peter Horvath (Light Microscopy Center of ETH Zurich) for precious help with image analysis. C.H. gratefully acknowledges financial support from the Professor Dr Max Cloëtta Foundation, ETH Zurich (TH-grant no. 05 07-3) and the Novartis Foundation of Medical and Biologic Research (Switzerland).
Authorship
Contribution: M.N. and C.H. planned the experiments and wrote the paper; M.N., D.A., and B.V. performed experiments and analyzed data; M.A. and R.L. designed and performed flow chamber experiments; M.L. and S.H. analyzed data; O.B. gave technical advice for the microscopy experiments; and H.L. and H.J.F. provided mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cornelia Halin, Institute of Pharmaceutical Sciences, ETH Zurich, Wolfgang-Pauli St 10, HCI H413, CH-8093 Zurich, Switzerland; e-mail: cornelia.halin@pharma.ethz.ch.