Abstract
The lymphatic vasculature preserves tissue fluid balance by absorbing fluid and macromolecules and transporting them to the blood vessels for circulation. The stepwise process leading to the formation of the mammalian lymphatic vasculature starts by the expression of the gene Prox1 in a subpopulation of blood endothelial cells (BECs) on the cardinal vein (CV) at approximately E9.5. These Prox1-expressing lymphatic endothelial cells (LECs) will exit the CV to form lymph sacs, primitive structures from which the entire lymphatic network is derived. Until now, no conclusive information was available regarding the cellular processes by which these LEC progenitors exit the CV without compromising the vein's integrity. We determined that LECs leave the CV by an active budding mechanism. During this process, LEC progenitors are interconnected by VE-cadherin–expressing junctions. Surprisingly, we also found that Prox1-expressing LEC progenitors were present not only in the CV but also in the intersomitic vessels (ISVs). Furthermore, as LEC progenitors bud from the CV and ISVs into the surrounding mesenchyme, they begin expressing the lymphatic marker podoplanin, migrate away from the CV, and form the lymph sacs. Analyzing this process in Prox1-null embryos revealed that Prox1 activity is necessary for LEC progenitors to exit the CV.
Introduction
Our understanding of the genes and mechanisms controlling the development of the mammalian lymphatic vasculature has greatly advanced in the last decade. The results of targeted inactivation experiments in mice have identified 3 transcription factors (Prox1, Sox18, and COUP-TFII) as crucial regulators of early lymphatic endothelial cell (LEC) differentiation in mammals; their functional inactivation results in a complete lack of LECs and, therefore, of the entire lymphatic vasculature.1-4 It is generally accepted that the initiation of Prox1 expression in a subpopulation of venous endothelial cells (VECs) in the wall of the mouse cardinal vein (CV) at around E9.5 is one of the earliest steps during mammalian lymphatic vasculature formation. We have previously shown that the embryonic veins are the unique source of the entire mammalian lymphatic vasculature by showing that Prox1-expressing LECs that exit the CV form the embryonic lymph sacs from which the entire lymphatic vasculature is eventually derived.2,5 Although it is known that in mammals Vegfc signaling is required for LECs to exit the CV,6 until now no conclusive data were available regarding the cellular mechanisms involved in this critical process. For instance, how do these endothelial cells (ECs) exit the CV? How is it that LECs leave the CV without disrupting the endothelial barrier? To address some of these important questions, we performed electron microscopy (EM) to visualize Prox1-expressing LEC progenitors leaving the CV. Results from these analyses determined that Prox1-expressing LEC progenitors leave the CV by budding and that the integrity of the CV is not compromised during this process. Unexpectedly, we also found Prox1-expressing LEC progenitors intermingled with the VECs lining the ISVs. These LEC progenitors also bud from the ISVs into the surrounding mesenchyme such that they meet and interconnect with the Prox1-expressing LEC progenitors that are budding from the CV. These migrating LECs will eventually form the lymph sacs. In mammals, the intersomitic vessels align dorsoventrally at the myotomal boundaries between somites.7,8 In zebrafish, venous intersomitic vessels form by the connection of primary dorsal-aorta–derived primary sprouts, and posterior cardinal vein–derived secondary sprouts. Lymphatic progenitors derived from the posterior cardinal vein will form the parachordal line from which all trunk lymphatics will be derived.9 In mammals, not much information is yet available regarding the origin or functional characteristics of the ISVs; that is, it is not yet clear whether they truly sprout from the CV or are generated by angioblasts.
We also reanalyzed Prox1-null embryos in which we previously reported that the Prox1 promoter activity detected outside the CV of those embryos indicated that Prox1 function was not required for LECs to exit the CV. The analysis we have now performed on thick sections of Prox1-null embryos reveals that the previously observed Prox1 promoter activity was not in ECs in the mesenchyme outside of the CV; instead its activity is localized inside the ISVs. This result argues that Prox1 activity is necessary for LECs to bud from the CV.
Significantly, this is the first report showing that Prox1-expressing LEC progenitors are also located in the ISVs, a result that will impact our current knowledge and interpretation of the early steps leading to the formation of the lymphatic vasculature in mammalian embryos.
Methods
Mice
Tie2Cre mice were provided by Dr M. Yanagisawa (University of Texas Southwestern Medical Center); R26YFP mice were obtained from The Jackson Laboratory. The methods for generating Prox1+/LacZ, COUP-TFIIflox/flox, and Prox1+/GFPCre mice were previoulsy reported.4,10,11 PlexinD1−/− embryos were previously described.12 All of the mouse experiments were approved by the St Jude Children's Research Hospital Animal Care and Use Committee.
Immunohistochemistry
For immunohistochemistry on vibratome sections, embryos were fixed in 4% paraformaldehyde (PFA) at 4°C overnight, embedded in 7% low melting point agarose, sectioned in a vibratome (100 μm), and immunostained. For immunohistochemistry on cryosections, embryos were fixed in 4% PFA at 4°C overnight, embedded in optimal cutting temperature (OCT) compound, sectioned in a cryostat (12 μm), and immunostained. The sections were mounted with mounting medium containing DAPI (Invitrogen), and confocal microscopy was performed.
Antibodies
The following primary antibodies were used: rabbit anti–β-gal (MP Biomedicals); rabbit anti-Prox1 (AngioBio); goat anti-Prox1 (R&D Systems); hamster anti-podoplanin (Hybridoma Bank); rat anti-Pecam1 (BD Pharmingen); guinea pig anti-Lyve1 (G.O., unpublished data, 2005); rabbit anti-Lyve1 (AngioBio); rat anti–VE-Cadherin (BD Pharmingen); and rabbit anti-GFP (Invitrogen). The following secondary antibodies were used: Alexa 488–conjugated donkey anti–rabbit (Invitrogen); Alexa 488–conjugated donkey anti–guinea pig (Invitrogen); Alexa 488–conjugated goat anti–hamster (Invitrogen); Alexa 488–conjugated donkey anti–goat (Invitrogen); Cy3-conjugated donkey anti–rabbit (Jackson ImmunoResearch Laboratories); Cy3-conjugated donkey anti–goat (Jackson ImmunoResearch Laboratories); and Cy5-conjugated donkey anti–rat (Jackson ImmunoResearch Laboratories).
Image analysis
Tissue sections prepared on a vibratome were immunostained with antibodies and then visualized with a confocal laser-scanning microscope (Zeiss LSM510). Subsequently, 3-D confocal stacks were reconstructed using ZEN 2011 software (Carl Zeiss). For images to be compared quantitatively the same intensity settings were used and images were acquired on the same day. The 3-D video was generated using IMARIS X64 6.2.1 software (Bitplane).
Electron microscopy
To detect β-gal activity in embryos, we performed X-gal staining as previously described but without using any detergent.13 Embryos were postfixed in 4% PFA overnight at 4°C and then cut into 200-μm thick vibratome sections. Sections were postfixed in 2% OsO4 for 1.5 hours at room temperature and stained in 2% uranyl acetate in the dark for 2 hours at 4°C. Then, sections were rinsed in distilled water, dehydrated in ethanol, and infiltrated overnight in Araldite (Durcupan; Fluka). After polymerization, embedded sections were detached from the chamber slide and glued to araldite blocks. Serial semithin (1.5 μm) sections were cut with an Ultracut UC-6 (Leica), mounted onto slides, and stained with 1% toluidine blue. Selected semithin sections were glued (Super Glue; Loctite) to araldite blocks and detached from the glass slide by repeated freezing (in liquid nitrogen) and thawing. Ultrathin (0.06-0.08 μm) sections were prepared with an Ultracut and stained with lead citrate. Finally, photomicrographs were obtained under a transmission electron microscope (FEI Tecnai G2 Spirit Biotwin) using a digital camera (Morada; Soft Imaging System, Olympus).
Statistical analysis
Microsoft Excel 2008 was used to evaluate the statistical significance by unpaired Student t test.
Results
Prox1-expressing LEC progenitors bud from the CV
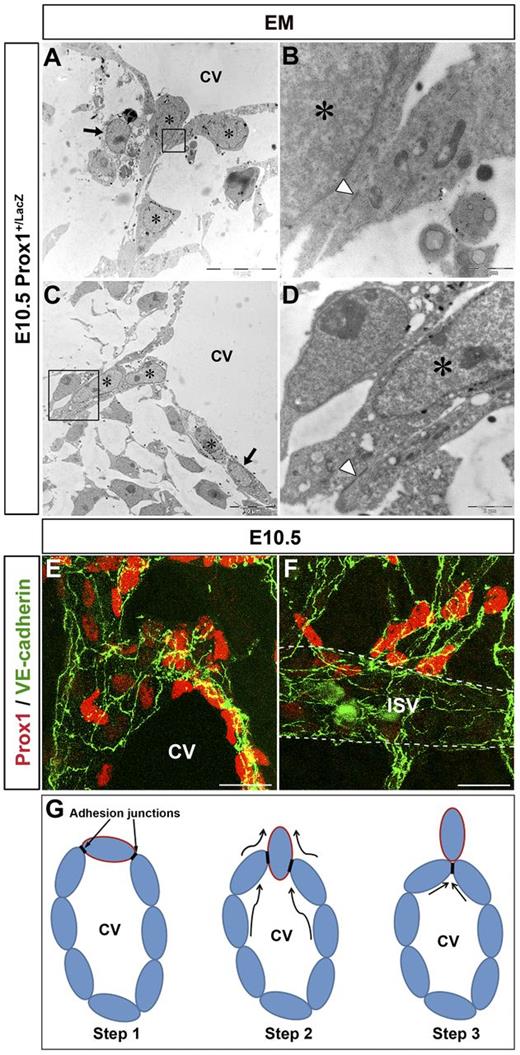
E10.5 Prox1+/LacZ embryos (n = 3 embryos) were stained with X-gal and processed for EM. The nuclei of Prox1-expressing cells were labeled by numerous small black aggregates near the nuclear envelope, facilitating their identification (Figure 1A-D stars; supplemental Figure 1A-B stars and black arrowheads, available on the Blood Web site; see the Supplemental Materials link at the top of the online article); negative cells were devoid of labeling (Figure 1A-C black arrows; supplemental Figure 1C). Using this approach, we conclusively determined that Prox1-expressing LEC progenitors exit the CV by a budding mechanism (Figure 1A-D) without compromising the integrity of the vein. During this process, Prox1-expressing LEC progenitors budded as an interconnected group of cells attached to each other and to the LEC progenitors in the CV by adhesion junctions (Figure 1B-D arrowheads). These junctions ensured that the venous integrity was not compromised during the budding process (Figure 1G).
Prox1-expressing LEC progenitors bud from the CV. (A-D) EM analysis of E10.5 Prox1+/LacZ embryos (n = 3 embryos) shows that Prox1-expressing cells (stars) exit the CV via an active budding mechanism. During this process, the budding cells are attached by cell junctions (arrowheads in panels B-D). LacZ negative cells are indicated by black arrows. Panels B and D are high-power magnifications of the black boxed area in panels A and C, respectively. (E-F) As the Prox1-expressing LEC progenitors bud off, they express high levels of the cell adhesion marker VE-cadherin (n = 5 embryos). (G) Diagramatic representation of the budding process of Prox1-expressing LEC progenitors from the CV (the border of LEC progenitors is painted in red). Adhesion junctions between the Prox1-expressing cells ensure the integrity of the cardinal vein during the budding process. CV indicates cardinal vein. Scale bars: 10 μm (A-C), 1 μm (B), 2 μm (D), and 20 μm (E-F).
Prox1-expressing LEC progenitors bud from the CV. (A-D) EM analysis of E10.5 Prox1+/LacZ embryos (n = 3 embryos) shows that Prox1-expressing cells (stars) exit the CV via an active budding mechanism. During this process, the budding cells are attached by cell junctions (arrowheads in panels B-D). LacZ negative cells are indicated by black arrows. Panels B and D are high-power magnifications of the black boxed area in panels A and C, respectively. (E-F) As the Prox1-expressing LEC progenitors bud off, they express high levels of the cell adhesion marker VE-cadherin (n = 5 embryos). (G) Diagramatic representation of the budding process of Prox1-expressing LEC progenitors from the CV (the border of LEC progenitors is painted in red). Adhesion junctions between the Prox1-expressing cells ensure the integrity of the cardinal vein during the budding process. CV indicates cardinal vein. Scale bars: 10 μm (A-C), 1 μm (B), 2 μm (D), and 20 μm (E-F).
It was previously shown that zipper-like cell-cell junctions precede the appearance of the button-like junctions characteristic of the quiescent and functional lymphatic capillary endothelium.14 More recently, these same authors reported that at E12.5, Prox1+ cells budding from the CV are joined by zipper-like junctions that are identified as continuous lines of VE-cadherin at the cell borders.15 To determine whether such zipper-like junctions are also present in the Prox1-expressing LEC progenitors in the beginning of the budding process at earlier stages, LECs were stained with antibodies against VE-cadherin. We found that Prox1-expressing LEC progenitors in the CV and those budding from the CV and ISVs were interconnected by VE-cadherin expressing zipper-like junctions at E10.5 (Figure 1E-F) and E11.5 (data not shown). The maintenance of high levels of VE-cadherin expression in LEC progenitors and in LECs that have budded off from the CV and ISVs helps preserve the adhesion junctions intact.
Intersomitic vessels are a newly identified source of Prox1-expressing LEC progenitors
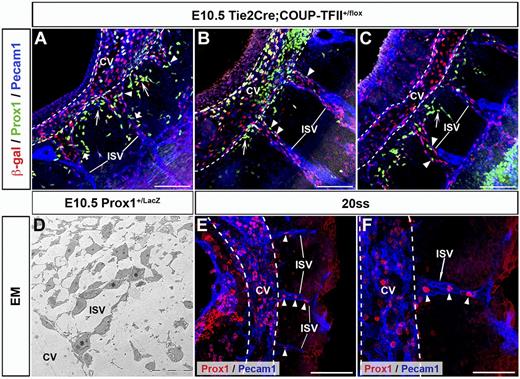
Detailed characterization of the early stages of lymphatic vasculature formation suggested that Prox1-expressing LEC progenitors normally detected on the wall of the CV leave the vein via specific migratory paths that extend radially from the dorsal half of the CV.2,4 To better characterize this process we now performed a detailed analysis of serial thick vibratome sections that retained the 3-D structure of the embryo. Using this approach we noticed that Prox1-expressing LEC progenitors were present not only in a polarized manner around the CV but were also present in the ISVs. The ISVs appear to be connected to the CV after they form and navigate between somites using the same cues that guide axon growth cones.12,16,17 Notably, although the vessel of origin for the ISV endothelium in mouse embryos remains controversial, these structures are already detected before the appearance of the first LEC progenitors in the CV.8,17 To further characterize the relationship between ISVs and Prox1-expressing LECs, we took advantage of available Tie2Cre;COUP-TFII+/f embryos in which COUP-TFII+ VECs can be visualized by β-gal staining after 1 copy of COUP-TFII is deleted without showing any obvious phenotype.10 Analysis of sagittal, frontal, and transverse sections revealed that Prox1-expressing LEC progenitors were not only present in the CV, but were also localized with COUP-TFII+ VECs inside all the ISVs at E10.5 (Figure 2A-C arrowheads; supplemental Figure 1D-F arrowheads; n = 3 embryos of each type of section).
Prox1-expressing LEC progenitors are also found on the ISVs. (A-C) Sagittal sections of E10.5 Tie2Cre;COUP-TFII+/f embryos (n = 3 embryos) showing the presence of Prox1-expressing LEC progenitors in the ISVs (arrowheads), which are labeled by COUP-TFII β-gal reporter staining. (A-C) Shown from anterior to posterior. Short arrows in panel A highlight the Prox1-expressing LEC progenitors that are budding off from the ISVs. Long arrows point to Prox1-expressing LEC progenitors budding off from various points around the CV that appear to alternate with those from the ISVs. (D) EM analysis showing that X-gal–positive Prox1-expressing cells (stars) are intermingled with X-gal–negative venous endothelial cells in the ISVs at E10.5. Higher magnification of the X-gal expressing cells is shown in supplemental Figure 1. (E-F) At the 20ss, Prox1-expressing LEC progenitors are already detected in the CV (white dashed line) and ISVs (arrowheads; n = 4 embryos). Panel F is high-power magnification of panel E. ISV, intersomitic vessels; Scale bars: 100 μm (A-C,E), 20 μm (D), and 50μm (F).
Prox1-expressing LEC progenitors are also found on the ISVs. (A-C) Sagittal sections of E10.5 Tie2Cre;COUP-TFII+/f embryos (n = 3 embryos) showing the presence of Prox1-expressing LEC progenitors in the ISVs (arrowheads), which are labeled by COUP-TFII β-gal reporter staining. (A-C) Shown from anterior to posterior. Short arrows in panel A highlight the Prox1-expressing LEC progenitors that are budding off from the ISVs. Long arrows point to Prox1-expressing LEC progenitors budding off from various points around the CV that appear to alternate with those from the ISVs. (D) EM analysis showing that X-gal–positive Prox1-expressing cells (stars) are intermingled with X-gal–negative venous endothelial cells in the ISVs at E10.5. Higher magnification of the X-gal expressing cells is shown in supplemental Figure 1. (E-F) At the 20ss, Prox1-expressing LEC progenitors are already detected in the CV (white dashed line) and ISVs (arrowheads; n = 4 embryos). Panel F is high-power magnification of panel E. ISV, intersomitic vessels; Scale bars: 100 μm (A-C,E), 20 μm (D), and 50μm (F).
At this stage, Prox1-expressing LEC progenitors are already budding off from the CV (Figure 2A-C long arrows). Strikingly, Prox1-expressing LEC progenitors were also budding off from the ISVs to migrate laterally into the mesenchymal space between ISVs (Figure 2A short arrows; supplemental Figure 1F arrow). In addition, EM analysis of E10.5 Prox1+/LacZ embryos showed that the ISVs were composed of a heterogeneous population of venous and lymphatic ECs (Figure 2D, supplemental Figure 1G-I).
Prox1-expressing LEC progenitors in the ISVs may correspond to progenitors that were initially present in the CV but later migrated into these vessels. Alternatively, these LEC progenitors could originate from the localized expression of Prox1 in the ISVs. At the moment, we cannot rule out either possibility. However, immunostaining of embryos from 20 somite-stage (ss), the earliest stage at which Prox1 expression can be detected in a subpopulation of VECs in the CV, shows Prox1-expressing LEC progenitors also present in the ISVs (Figure 2E-F arrowheads; n = 3 embryos). There were no LEC progenitors located either in the CV or ISVs before this stage (data not shown). This result shows that Prox1-expressing LEC progenitors are present in the CV and ISVs concomitantly from the earliest stages of LEC differentiation. In addition, the 2 key transcription factors shown to be the direct regulators of Prox1 expression in the CV, (COUP-TFII; Figure 2) and Sox1811 are also expressed in the ISVs. To further characterize the contribution of ISVs to lymphatic formation, live imaging would have been a useful approach. Unfortunately, as mouse embryos are relatively opaque, deep tissue live imaging of the early steps of lymphatic vascular formation is still technically challenging. Therefore, we took advantage of PlexinD1 mutant embryos. It was previously shown that plexinD1 loss-of-function mutations in mice and zebrafish result in patterning defects of ISVs; these defects are not because of changes in EC proliferation.12,17 In particular, analysis of PlexinD1−/− mouse embryos revealed obvious alterations in the patterning of the ISVs as well as a general increase in the number of ISV branches at E10.5 (supplemental Figure 2). In agreement with our proposal that ISVs are also a source of LEC progenitors, in PlexinD1−/− embryos we found Prox1+ cells located in the ISVs branches as well as a general increase in the number of LEC progenitors in the mesenchyme surrounding the ISVs (supplemental Figure 2A-B arrowheads, C; n = 3 embryos, *P < .05).
Podoplanin expression provides the first molecular distinction between LEC progenitors inside the CV and ISVs and those outside these structures
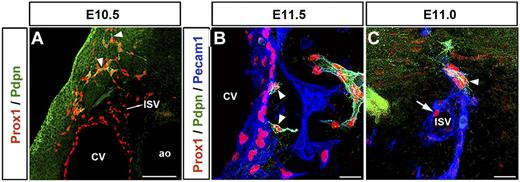
Unlike other lymphatic EC markers, such as Prox1 and Lyve1, podoplanin expression was only detected in the migrating front of the Prox1-expressing LECs after these cells were out of the CV; in the anterior region of the embryo this starts to be detected at around E10.5 (Figure 3A arrowheads; n = 5 embryos). Podoplanin expression cannot be detected in the Prox1-expressing LECs outside of the CV before E10.5 (data not shown). In fact, in agreement with previous reports,18 extensive podoplanin expression in LECs along the anterior-posterior axis was only seen after Prox1-expressing LECs fully exited the CV at approximately E11.5 (Figure 3B arrowheads; supplemental Figure 3A-B; n = 5 embryos). Likewise, Prox1-expressing LEC progenitors inside the ISVs were podoplanin-negative (Figure 3C arrow; supplemental Video 1); podoplanin expression occurred after LEC progenitors left the ISVs (Figure 3C arrowhead; n = 5 embryos; supplemental Video 1). This pattern suggests that Prox1-expressing LECs in the ISVs have the same molecular identity (LEC progenitors) as those in the CV. It also argues that the expression of podoplanin provides the first molecular distinction between the Prox1+/podoplanin− LEC progenitors inside the CV and ISVs and the Prox1+/podoplanin+ LECs outside these structures.
LEC differentiation occurs outside the CV and ISVs. (A) In the anterior region of the embryo, the lymphatic marker podoplanin starts to be expressed in the migrating front of Prox1-expressing LECs (arrowheads) outside the CV at approximately E10.5. The Prox1+podoplanin− cells seen outside the CV are located in the ISVs. (B) Extensive podoplanin expression is only detected after Prox1-expressing LEC progenitors fully exit the CV at around E11.5 (arrowheads). (C) Similarly, Prox1-expressing cells on the ISV are negative for podoplanin (arrow), which is expressed as soon as those cells bud off (arrowhead; n = 5 embryos of each stage). Scale bars: 100 μm (A), 20 μm (B-C).
LEC differentiation occurs outside the CV and ISVs. (A) In the anterior region of the embryo, the lymphatic marker podoplanin starts to be expressed in the migrating front of Prox1-expressing LECs (arrowheads) outside the CV at approximately E10.5. The Prox1+podoplanin− cells seen outside the CV are located in the ISVs. (B) Extensive podoplanin expression is only detected after Prox1-expressing LEC progenitors fully exit the CV at around E11.5 (arrowheads). (C) Similarly, Prox1-expressing cells on the ISV are negative for podoplanin (arrow), which is expressed as soon as those cells bud off (arrowhead; n = 5 embryos of each stage). Scale bars: 100 μm (A), 20 μm (B-C).
Podoplanin-expressing specified LECs migrate as an interconnected group of cells
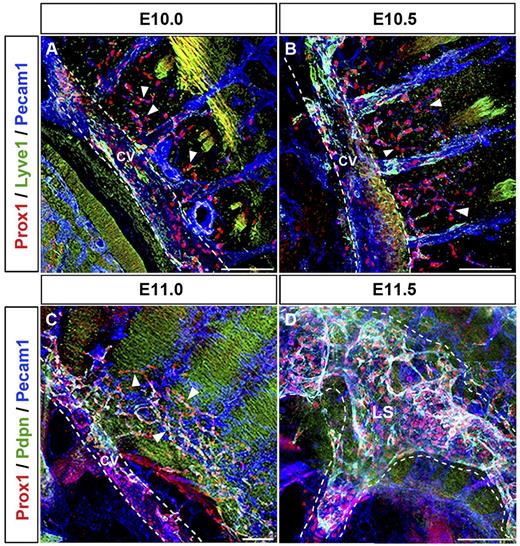
To better characterize the process of lymph sac formation, we next analyzed thick sagittal sections of embryos isolated between E9.5 and E12.5 (n = 4 embryos of each stage). Prox1-expressing LEC progenitors that budded off from the CV migrated dorsally into the surrounding mesenchymal tissue starting at approximately E10.0 (Figure 4A arrowheads). During this migration, Prox1-expressing LECs remained interconnected to each other and the Prox1-expressing cells that budded off from the CV and those that budded off from the ISV merged together at around E10.5 (Figure 4B arrowheads). Around this stage, fewer Prox1-expressing LECs have budded off from the posterior region of the embryo (compare with the anterior region shown in Figure 4B) and they are still negative for podoplanin expression (Figure 5A arrowheads; compare with podoplanin expression in the anterior region shown in Figure 3A). At around E11.0, this initial cluster of migrating LECs formed a capillary-like structure along the anterior and posterior axis of the embryo (Figures 4C and 5B arrowheads). After further sprouting and migration this capillary-like structure organized into a lymph sac-like structure at approximately E11.5 (supplemental Figures 3C-D, 4D, and 5C) that become more defined at E12.5 (Figure 5D).
Prox1-expressing LECs migrate as an interconnected group of cells. (A) Sagittal sections along the anterior region of the embryo showing that Prox1-expressing LEC progenitors that bud off from the CV (white dashed line) and ISVs migrate dorsally and longitudinally (arrowheads) into the surrounding mesenchymal tissue starting at approximately E10.0. (B) Prox1-expressing LECs remain interconnected during migration into the surrounding mesenchyme (arrowheads), and Prox1-expressing LECs from the CV and the ISVs eventually merge together at E10.5. (C) Migrating LECs that have been merging together at earlier stages form a capillary-like structure (arrowheads) at approximately E11.0 in the anterior region. (D) Prox1-expressing LECs organize into a lymph sac-like structure (white dashed line) at approximately E11.5 after further sprouting and migration. LS, lymph sac (n = 4 embryos of each stage). Scale bars: 100 μm (A-D).
Prox1-expressing LECs migrate as an interconnected group of cells. (A) Sagittal sections along the anterior region of the embryo showing that Prox1-expressing LEC progenitors that bud off from the CV (white dashed line) and ISVs migrate dorsally and longitudinally (arrowheads) into the surrounding mesenchymal tissue starting at approximately E10.0. (B) Prox1-expressing LECs remain interconnected during migration into the surrounding mesenchyme (arrowheads), and Prox1-expressing LECs from the CV and the ISVs eventually merge together at E10.5. (C) Migrating LECs that have been merging together at earlier stages form a capillary-like structure (arrowheads) at approximately E11.0 in the anterior region. (D) Prox1-expressing LECs organize into a lymph sac-like structure (white dashed line) at approximately E11.5 after further sprouting and migration. LS, lymph sac (n = 4 embryos of each stage). Scale bars: 100 μm (A-D).
Lymph sacs form between E11.5 and E12.5. (A) Sagittal section of the posterior region of an E10.5 embryo revealed that at this early stage fewer Prox1-expressing LECs budded off from CV and ISVs (arrowheads) in the posterior region of the embryo (compare with anterior region in Figure 4B). These Prox1-expressing LECs are negative for podoplanin (compare with podoplanin expression in the anterior region shown in Figure 3A). (B) At approximately E11.0, migrating LECs form a capillary-like structure (arrowheads) also in the posterior area. (C) Prox1-expressing LECs organize into a lymph sac-like structure (white dashed line) at approximately E11.5. (D) The lymph sac (white dashed line) was obvious at approximately E12.5. All are sagittal sections (100-μm thick). LS, lymph sac (n = 4 embryos of each stage). Scale bars: 100 μm (A-D).
Lymph sacs form between E11.5 and E12.5. (A) Sagittal section of the posterior region of an E10.5 embryo revealed that at this early stage fewer Prox1-expressing LECs budded off from CV and ISVs (arrowheads) in the posterior region of the embryo (compare with anterior region in Figure 4B). These Prox1-expressing LECs are negative for podoplanin (compare with podoplanin expression in the anterior region shown in Figure 3A). (B) At approximately E11.0, migrating LECs form a capillary-like structure (arrowheads) also in the posterior area. (C) Prox1-expressing LECs organize into a lymph sac-like structure (white dashed line) at approximately E11.5. (D) The lymph sac (white dashed line) was obvious at approximately E12.5. All are sagittal sections (100-μm thick). LS, lymph sac (n = 4 embryos of each stage). Scale bars: 100 μm (A-D).
Prox1 is essential for LEC progenitors to leave the CV
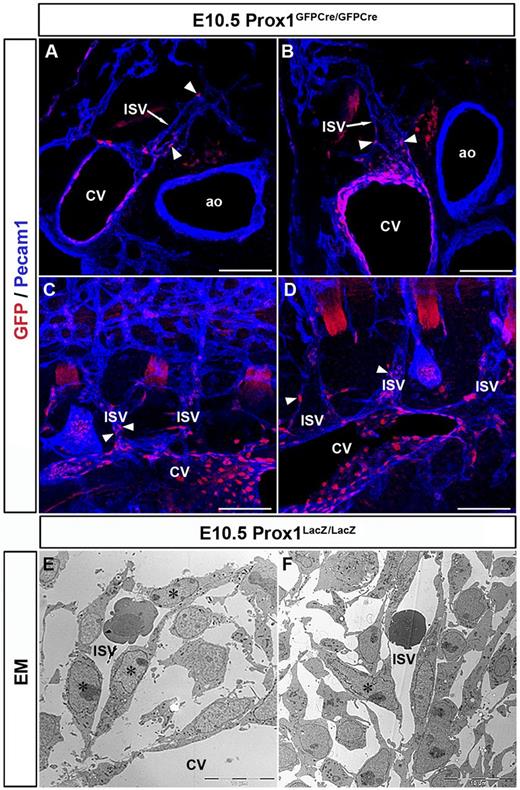
Compared with wild-type (WT) embryos, Prox1+/− embryos have significantly fewer Prox1-positive cells within and outside the CV19 (supplemental Figure 4). Originally, we reported that β-gal+ cells of Prox1LacZ/LacZ embryos exit the CV at E10.5,4 suggesting that Prox1 activity was not required for LEC progenitors to leave the CV. However, considering our finding that LEC progenitors are present not only in the CV but also in the ISVs, we decided to re-evaluate our original interpretation by examining thick sections of Prox1-null embryos in which the entire structure of the ISVs was retained. Immunostained transverse and sagittal vibratome sections (100 μm) of E10.5 Prox1GFPCre/GFPCre embryos (which display the same phenotype as the original Prox1LacZ/LacZ embryos)11 showed GFP+ cells (these cells represent cells in which the Prox1 promoter was activated, but no functional protein was produced) only in the CV and ISVs of these embryos (Figure 6A-D arrowheads, n = 3 embryos). Unlike in WT littermates or heterozygous littermates, null embryos had no GFP+ cells budding from the CV or ISVs (Figure 6A-D). These data indicated that the LacZ+ cells observed on thin sections and that we previously thought exited the CV in the absence of Prox1 activity were actually located in the ISVs. Furthermore, EM analysis of E10.5 Prox1LacZ/LacZ embryos showed that LacZ+ cells are inside the blood-containing ISVs (Figure 6E-F stars) suggesting that LEC progenitors fail to leave the CV and ISVs in Prox1-null embryos and that Prox1 activity is required during this process.
Prox1 is essential for LEC progenitors to leave the CV. Transverse sections (A-B) and sagittal sections (C-D) from E10.5 Prox1GFPCre/GFPCre embryos (Prox1 null) showing that GFP-positive cells outside the CV are all located in the ISVs (arrowheads) and not in the surrounding mesenchyme (n = 3 embryos). (E-F) EM analysis showing that X-gal+ cells (stars) are colocalized with the venous ECs in the ISV outside the CV in E10.5 Prox1LacZ/LacZ embryos. Scale bars: 100 μm (A-D), 10 μm (E-F).
Prox1 is essential for LEC progenitors to leave the CV. Transverse sections (A-B) and sagittal sections (C-D) from E10.5 Prox1GFPCre/GFPCre embryos (Prox1 null) showing that GFP-positive cells outside the CV are all located in the ISVs (arrowheads) and not in the surrounding mesenchyme (n = 3 embryos). (E-F) EM analysis showing that X-gal+ cells (stars) are colocalized with the venous ECs in the ISV outside the CV in E10.5 Prox1LacZ/LacZ embryos. Scale bars: 100 μm (A-D), 10 μm (E-F).
Discussion
Because of the obvious technical difficulties that still preclude the use of live imaging in later-stage mouse embryos, in this study we used a detailed immunologic analysis on thin and semithick serial sections to characterize and reconstruct the earliest steps of mammalian lymphangiogenesis. In one of her seminal works, Florence Sabin proposed that the fact that the first lymph sacs lie close to the vein and are separated only by the double endothelial wall suggests that lymphatics budded directly from the veins and that these buds unite to form plexuses that develop into sacs.20 We have now confirmed that once again her statement is accurate by showing that in mammals, LEC progenitors leave the CV by an active budding mechanism. We also show that as they leave these cells they remain attached to each other by adhesion junctions. There are many examples of collective cell migrations that occur with maintenance of cell-cell junctions (eg, cell migrations in the lateral line of zebrafish). Collective cell migration takes place when cells that are maintaining cell contacts form a unit that moves through tissues during morphogenesis, vascular sprouting, and so on. All of this depends on collective cell adhesion dynamics and the integrity of the cell junctions. As LECs leave the vein, those ECs remaining in the CV will probably divide and elongate in such a manner that the integrity and caliber of the vein will be maintained. At the same time that LECs are budding off from the CV, the adhesion junctions will be moving along those cells in such a way that the CV integrity is never compromised (Figure 1G). Then, as LECs leave the vein, the adjacent ECs in the CV get closer to each other and establish new adhesion junctions that will keep the CV sealed (Figure 1G step 3). Concomitantly, the primary lymph sacs will start to form in the surrounding mesenchyme, and previous results provided anatomical evidence of connections between the forming lymph sacs and the CV.20-22 Platelets were proposed to be required to seal the separation of the forming lymph sacs from the CV at those connecting points.23,24 However, the budding mechanism we report on this paper also suggests that the role of platelet microthrombi in mediating blood-lymphatic vascular separation25 might be restricted to the region where lymphatics and blood vessels coalesce, the putative lymphovenous valves.19
The ISVs align dorsoventrally at the myotomal boundaries between somites.7,8 Our results showing that the ISVs provide an additional source of LEC progenitors help explain the large and fast increase in the number of Prox1-expressing LECs migrating along the mesenchymal tissue leading to the formation of the primitive lymph sacs. Recently, a ballooning process was proposed to explain the process of lymph sac formation18 ; however, our detailed analysis failed to visualize these type of structures. Our current data add important information to this process by showing that the ISVs are another important source of LEC progenitors and that Prox1 activity is required for LECs to bud from the CV. Furthermore, this result also suggests that despite the numerous differences in the process of lymphatic vessels formation between zebrafish and mammals (eg, fish lack lymph sacs), the process of LEC migration in mice may be closer to that of zebrafish than previously thought; in zebrafish venous sprouts and LEC precursors move out from the CV at the same time.7,9
This analysis also allowed us to reinterpret our previous data suggesting that Prox1 activity was not required for LECs to exit the CV.4 In that work, we analyzed X-gal–stained Prox1-null embryos, observed LacZ+ cells outside the CV, and argued that Prox1 function was required for LEC specification but was not required for those cells to leave the CV. As shown now in Figure 6A through D, those GFP+ cells outside the CV are inside the ISVs, demonstrating that Prox1 activity is also required during the budding process.
Our data suggest that at least 2 steps are involved in the early maturation of LECs in mammals: an initial step mediated by Prox1 in the CV and ISVs by which a subpopulation of VECs become LEC progenitors, and a subsequent step highlighted by the expression of podoplanin (ie, LEC specification) that occurs as soon as LECs bud from the CV and ISVs. The finding that podoplanin expression only starts after LEC progenitors exit the CV highlights a molecular distinction between venous Prox1-expressing LEC progenitors and specified podoplanin-expressing LECs outside the CV (see model in Figure 7). Although most Prox1-expressing LEC progenitors will eventually exit the veins, acquire the expression of additional LEC markers, and further mature, a smaller subpopulation will remain in the veins and participate in the formation of the lymphovenous valves.19
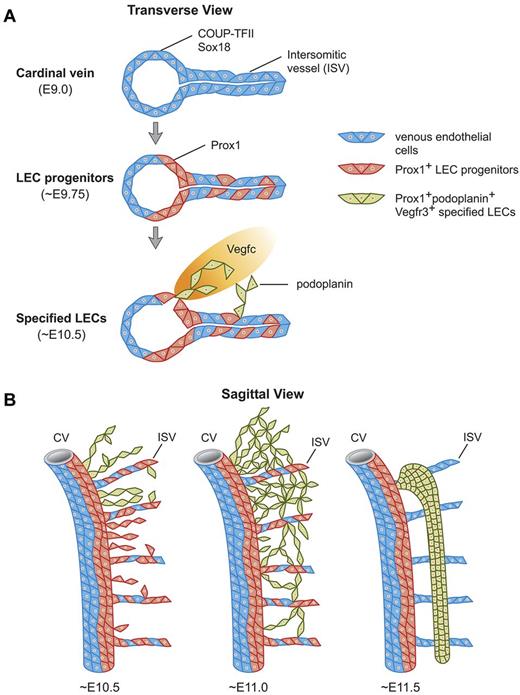
Stepwise development of the mammalian lymph sacs. (A) Diagramatic representation of a transverse view of the CV of a mouse embryo showing that at ∼ E9.0, COUP-TFII, and Sox18 are expressed in VECs in the CV and ISVs. Sox18 and COUP-TFII expression induce Prox1 expression at ∼ E9.75 in a subset of competent VECs both in the CV and ISVs. These Prox1-expressing cells become LEC progenitors. Around E10.5 and as these LEC progenitors bud off from the CV and ISVs under the influence of mesenchymal Vegfc signals, they start to express podoplanin. Once LEC progenitors exit the CV and start expressing podoplanin, the LEC fate becomes specified. (B) Temporal representation of this process on sagittal sections. At E10.5 the number of LEC progenitors that budded off from the CV and ISVs is more abundant in the anterior region of the embryo and Prox1-expressing LECs in the migrating front start to express podoplanin around this stage. Around E11.0, all Prox1-expressing LEC progenitors outside of the CV and ISVs express podoplanin. As LECs bud off from the CV dorsally and from the ISVs longitudinally, they merge together in the mesenchymal tissue to form a capillary-like structure at E11.0 that is more obvious in the anterior region of the embryo. At around E11.5, these specified podoplanin-expressing LECs organize into a lymph sac-like structure.
Stepwise development of the mammalian lymph sacs. (A) Diagramatic representation of a transverse view of the CV of a mouse embryo showing that at ∼ E9.0, COUP-TFII, and Sox18 are expressed in VECs in the CV and ISVs. Sox18 and COUP-TFII expression induce Prox1 expression at ∼ E9.75 in a subset of competent VECs both in the CV and ISVs. These Prox1-expressing cells become LEC progenitors. Around E10.5 and as these LEC progenitors bud off from the CV and ISVs under the influence of mesenchymal Vegfc signals, they start to express podoplanin. Once LEC progenitors exit the CV and start expressing podoplanin, the LEC fate becomes specified. (B) Temporal representation of this process on sagittal sections. At E10.5 the number of LEC progenitors that budded off from the CV and ISVs is more abundant in the anterior region of the embryo and Prox1-expressing LECs in the migrating front start to express podoplanin around this stage. Around E11.0, all Prox1-expressing LEC progenitors outside of the CV and ISVs express podoplanin. As LECs bud off from the CV dorsally and from the ISVs longitudinally, they merge together in the mesenchymal tissue to form a capillary-like structure at E11.0 that is more obvious in the anterior region of the embryo. At around E11.5, these specified podoplanin-expressing LECs organize into a lymph sac-like structure.
It has already been shown that podoplanin mutant embryos have blood-filled lymphatics, and that its function is mostly related to platelet aggregation in the CV. Podoplanin is a lymphatic endothelial surface protein that binds the CLEC-2 receptors on platelets and was previously shown to promote EC migration, adhesion, and tube formation in cultured cells.26 More recently, Osada et al proposed that during embryonic development, podoplanin in LECs activates platelets by binding to CLEC-2 in the connection between lymph sacs and CV and that inhibits migration, proliferation, and tube formation, which facilitates blood/lymphatic vessel separation.27 In any case, our description that podoplanin expression only starts after LECs leave the CV is probably an indication of the LEC maturation process. Our findings emphasize the idea that the process of LEC differentiation is a stepwise process that starts in the veins but continues after the LEC progenitors leave the CV.
In summary, our results reveal how Prox1-expressing cells leave the CV during early lymphangiogenesis and provide mechanistic insight into the stepwise process leading to LEC fate maturation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. Tsai and Dr M.-J. Tsai for providing the COUP-TFII floxed mouse strain, and the St Jude Tissue Imaging Center for their help with confocal imaging. The authors also thank Klo Spelshouse from Biomedical Communications for her help in the design of Figure 7. They thank Dr Cherise Guess for editing the paper, and all of the members of the Oliver laboratory for their helpful suggestions and discussions.
This work was supported by National Institutes of Health grant R01-HL073402 (to G.O.) and the American Lebanese Syrian Associated Charities (ALSAC).
National Institutes of Health
Authorship
Contribution: Y.Y. designed and performed the research, analyzed the data, and contributed to the paper preparation; J.M.G.-V. and M.S.-N. performed the EM analysis; R.S.S. and J.S. provided valuable discussions and ideas; M.K.S. and J.A.E. provided the PlexinD1 mutant embryos; and G.O. contributed to the experimental design, discussions, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guillermo Oliver, Dept of Genetics, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105-3678; e-mail: guillermo.oliver@stjude.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal