In this issue of Blood, Holien et al report that MYC addiction is responsible for rapid death of myeloma cell lines and primary myeloma tumor cells treated with a specific MYC inhibitor.1
The Myc family, which includes MYC, MYCN, and MYCL, are bHLHZip transcription factors that have a key role in diverse intracellular processes, including cell growth, metabolism, protein translation, ribosome biogenesis, apoptosis, and differentiation, as well as extracellular processes affecting the local microenvironment.2,3 Recent evidence indicates that MYC is a universal nonlinear amplifier of most expressed genes, but does not selectively determine which genes are expressed (Nie Z, Hu G, Wei G, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells, manuscript submitted, 2012). MYC, one of the first oncogenes identified in human cancer, is dysregulated or overexpressed in most kinds of human cancer cells as a result of genomic changes or upstream oncogenic signals.2,3 It appears that distinct expression thresholds determine the role of MYC in oncogenesis.3 Dysregulated expression at low levels can cause limited ectopic proliferation, but slightly higher levels of “oncogenic” expression activate the ARF/TP53 and apoptosis tumor suppressor pathways that limit the effect of MYC in the absence of other oncogenic events. Dysregulated overexpression of MYC from a transgene results in sporadic tumors in animal models, a result that is facilitated by inactivation of TP53 or apoptotic mechanisms. Using an omomyc transgene that binds to all Myc family members to inhibit formation of functional heterodimeric complexes with MAX, MYC was shown to be required for both proliferation and survival of cancer cells. Inhibition of MYC also had profound effects on regenerating tissues, but the effects were well tolerated for long periods, and were reversible when MYC function was no longer inhibited. Therefore, intermittent inhibition of MYC seems to have therapeutic potential in many kinds of tumors.
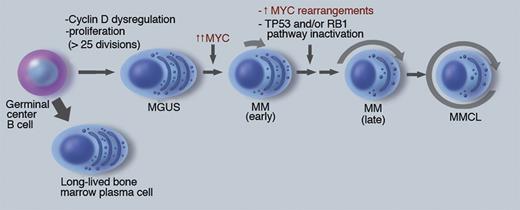
There is increasing evidence that MYC has a critical albeit incompletely understood role in the pathogenesis of multiple myeloma (MM; see figure).4-6 Initiating oncogenic events, including the nearly invariant dysregulation of a Cyclin D gene, are thought to occur in a germinal center B cell, and result in a premalignant monoclonal gammopathy of undetermined significance (MGUS) tumor. A detectable MGUS tumor has at least 108 cells—indicating more than 25 preceding cycles of proliferation—but less than 1% of plasma cells (PCs) are Ki67 positive. Progression to MM is associated with a several-fold increase in MYC RNA expression, the mechanism of which is poorly understood but sometimes might be due to genomic rearrangements. Newly diagnosed MM tumors are minimally proliferative, typically with 2% to 10% Ki67 positive tumor cells, suggesting that other consequences of MYC dysregulation may be important at this stage. In addition to an increased tumor mass and its consequences, progression of MM can include increased proliferation, decreased apoptosis, and decreased BM stromal cell–dependence. Multiple myeloma cell lines (MMCLs), which are mostly derived from advanced extramedullary tumor, nearly always have inactivated the TP53 pathway and more than 85% have genomic rearrangements in the MYC locus. The critical role of MYC in the transition from MGUS to MM is strongly supported by the Vk*MYC mouse model, in which the sporadic activation of MYC by somatic hypermutation results in the invariant occurrence of MM in an MGUS-prone mouse strain that rarely develops MM.7
The role of MYC in pathogenesis of multiple myeloma. MM pathogenesis is depicted as a linear pathway that appears to be initiated in a germinal center B cell, with subsequent transformation to a premalignant MGUS tumor that is stable but can sporadically progress to early MM, and then late MM, from which MM cell lines sometimes can be generated. The approximate fraction of cells actively cycling is depicted by length of arrow surrounding the cells. The dark nuclei reflect the antigen selection of cells after somatic hypermutation and IgH switch recombination. Some key pathogenic events are indicated, with further information in the text and references. Professional illustration by Marie Dauenheimer.
The role of MYC in pathogenesis of multiple myeloma. MM pathogenesis is depicted as a linear pathway that appears to be initiated in a germinal center B cell, with subsequent transformation to a premalignant MGUS tumor that is stable but can sporadically progress to early MM, and then late MM, from which MM cell lines sometimes can be generated. The approximate fraction of cells actively cycling is depicted by length of arrow surrounding the cells. The dark nuclei reflect the antigen selection of cells after somatic hypermutation and IgH switch recombination. Some key pathogenic events are indicated, with further information in the text and references. Professional illustration by Marie Dauenheimer.
Holien et al showed that 10058-F4, an effective inhibitor of heterodimerization of MAX with MYC (but not with MYCN or MYCL) and transcriptional activation by MYC, caused a substantial and rapid decrease in viability of 5 MMCLs that express MYC, but had no effect on a MMCL that expresses MYCL but not MYC.1 Supportively, introduction by lentiviral transfection of MYC-specific short hairpin RNA into MMCLs resulted in decreased MYC RNA and decreased viability in all 3 MMCLs tested. They also showed a substantial decrease in viability of cells from 12 primary MM tumors that were cultured for 72 hours with 10058-F4. The rapid loss of cell viability suggests that MYC is required not only for proliferation but also for survival of MMCLs and cultured primary MM tumor cells. Although additional studies are needed to confirm MYC addiction in MM tumors and MMCLs, it is important to note that 10058-F4 is not suitable for use in vivo.
Recently two groups reported that JQ1, which targets BET bromodomains on chromatin proteins (eg, BRD4, which regulates transcription elongation), inhibits the proliferation and survival of MMCLs and primary MM tumors, but also growth of murine MM tumors in the Vk*MYC model.8,9 Both groups suggested that JQ1-mediated inhibition of MM proliferation and survival was due to an inhibition of MYC expression. However, it is apparent that JQ1 does not uniquely target MYC, and it may be premature to conclude that the observed effects of JQ1 are mediated mainly by an effect on MYC.10 Regarding the potential MYC addiction of MM tumor cells, it was reported that IRF4 addiction of MMCLs may be due, at least partially, to IRF4-dependent MYC expression; this group also showed that a knockdown of MYC RNA in MMCLs resulted in decreased proliferation but with no significant effect on survival after 48 hours.11
Clearly, we are at the beginning of an effort to show that MYC addiction has a useful therapeutic potential for MM tumors. Although it is important to study MYC addiction in the Vk*MYC mouse model for early- and late-stage MM tumors, there are other questions that still require answers. First, we need to have a better understanding regarding the molecular characteristics of tumors that are effectively eliminated by inhibitors of MYC. Second, at what stages of tumorigenesis (eg, MGUS vs early and late intramedullar vs extramedullary) are MM tumors susceptible to MYC inhibitors? Third, is it possible to develop therapeutic strategies that will specifically inhibit MYC function? Fourth, will BET inhibitors fulfill this role given that it is unlikely that they specifically target MYC? Finally, will the inhibition of MYC function have as little effect in humans as seems to be the case in mice, and what are the time limits regarding how long individuals can tolerate MYC inhibition of normal tissues? So, despite the promise, much remains to be done.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal