Abstract
Platelets are vital for hemostasis because they release their granule contents in response to vascular damage. Platelet exocytosis is mediated by soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs), whose interactions are governed by regulators, eg, Sec/Munc18 proteins. These proteins chaperone syntaxin t-SNAREs and are required for exocytosis. Platelets contain 3 Munc18 isoforms: Munc18a, Munc18b, and Munc18c. We report that Munc18b is the major isoform and is required for platelet secretion. Familial hemophagocytic lymphohistiocytosis type 5 (FHL5) is caused by defects in the Munc18b/STXBP2 gene. We confirm a previous report showing that platelets from FHL5 patients have defective secretion. Serotonin, ADP/ATP, and platelet factor 4 release was profoundly affected in the 2 biallelic patients and partially in a heterozygous patient. Release of lysosomal contents was only affected in the biallelic platelets. Platelets from the FHL5 biallelic patients showed decreased Munc18b and syntaxin-11 levels were significantly reduced; other syntaxins were unaffected. Munc18b formed complexes with syntaxin-11, SNAP-23, and vesicle-associated membrane protein-8 in human platelets. Other potential secretion regulators, Munc13-4 and Rab27, were also found associated. These data demonstrate a key role for Munc18b, perhaps as a limiting factor, in platelet exocytosis and suggest that it regulates syntaxin-11.
Introduction
Circulating platelets play a key role in hemostasis and its sequelae. In vascular injury, platelets adhere to the damaged site, become activated, and secrete their granule contents. There are 3 granule classes in platelets: α-granules, dense granules, and lysosomes. Proteomic studies show that platelets contain and secrete hundreds of bioactive molecules that are important for vascular integrity. For example, α-granules contain molecules (eg, fibrinogen, PF4, factor V) that are important for platelet adhesion and aggregation, clot formation, wound healing, angiogenesis, and inflammation.1,2 Dense granules contain small molecules that are important for promoting platelet activation (eg, serotonin, ADP, and Ca2+).3 Lysosomes contain hydrolytic enzymes (eg, β-hexosaminidase), which may contribute to thrombus remodeling.4 Given its importance, it is critical to understand platelet exocytosis at a mechanistic level because elements of the secretory process may prove to be viable therapeutic targets for controlling thrombosis.
The platelet release reaction is mediated by soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs) and SNARE regulators.5 There are 2 general classes of SNARE proteins: target membrane SNAREs (t-SNAREs) and granule/vesicle-associated SNAREs (v-SNAREs). t-SNAREs and v-SNAREs form a complex that spans the 2 fusing bilayers (target and granule membrane); it is that complex that is minimally required for membrane fusion and subsequent granule cargo release.6 Several v- (ie, vesicle-associated membrane protein [VAMP]-2, -3, -7, -8) and t-SNAREs (ie, synaptosomal-associated protein [SNAP]-23, -25, syntaxin-2, -4, -7, -11, -13) are present in platelets (reviewed in Ren et al5 ). Among the v-SNAREs, Ren et al showed that VAMP-8 is required for release from the 3 granules.7 Of the 2 types of t-SNAREs, in several studies authors have described the importance of SNAP-23.8-10 This class is anchored to membranes via thioester-linked palmitates. Recent work has clarified the roles of the syntaxins, showing that syntaxin-11 is required for platelet secretion.11 Despite being the core machinery, essential for membrane fusion, SNAREs need regulators to control when and where they interact.
Sec/Munc18 (SM) proteins are a conserved family of cytosolic proteins that are required for most intermembrane trafficking events and cellular exocytosis. They are syntaxin-specific chaperones that may contribute to matching cognate v- and t-SNARE, thus forming fusogenic, trans-bilayer complexes.12 In mammals, there are 7 SM proteins, and each has been assigned to specific types of membrane-trafficking events: Munc18a, Munc18b, and Munc18c for exocytosis; Sly1 for protein biosynthesis; Vps45 for endocytosis; and Vps33a and Vps33b for protein degradation.13 Munc18a (also called STXBP1, Munc18-1, or nSec1) originally was identified in neurons as a syntaxin-1 chaperone.14 It plays an important role in neurotransmission and neuroendocrine release. Deletion of Munc18a causes a decrease in syntaxin-1.15-17 Munc18b (also called STXBP2 or Munc18-2) is broadly expressed and has been shown to interact with syntaxin-1A, -1B, -2, and -3.12 Munc18b plays a role in exocytosis from mast cells,18 neutrophils,19 and natural killer (NK) cells.20,21 Munc18c (also called STXBP3, PSP, or Munc18-3) is ubiquitously expressed22,23 and can interact with both syntaxin-2 and -4. The interaction between Munc18c and syntaxin-4 is important for insulin release from adipose tissue and muscle cells and is important in endothelial cell activation.24-26
In human and mouse platelets, 3 Munc18 isoforms are present: Munc18a, Munc18b, and Munc18c.27,28 Using inhibitory peptides (based on a potential regulatory site in Munc18s: Pep3) or anti-Munc18 antibodies, our group showed that at least one Munc18 isoform is important for platelet secretion.28 In permeabilized platelets, the Pep3 peptide inhibited release from all 3 granules classes. Using a monoclonal anti-Munc18c antibody and a cocktail of inhibitory peptides that disrupted Munc18c–syntaxin-4 interactions, Houng et al showed the potential importance of Munc18c–syntaxin-4 interactions.27 In addition, both Munc18a and 18c are phosphorylated on platelet stimulation, resulting in reduced affinity for their cognate syntaxins.27,28 The relevance of this finding has not been established. Despite the apparent importance of Munc18c, platelets from Munc18c heterozygous mice, which had a ∼ 30% reduction in the protein, showed no secretion defect.29 These divergent results suggest that more information is required before assigning function to the platelet Munc18 isoforms.
Familial hemophagocytic lymphohistocytosis (FHL) is a life-threatening genetic disease resulting from cytotoxic T lymphocytes and NK cell dysfunction. It manifests as fever, splenomegaly, and lymphocytosis.30,31 Five defective loci are associated with FHL; the 4 identified genes are: FHL2/perforin, FHL3/Munc13-4/Unc13D, FHL4/syntaxin-11, and FHL5/Munc18b/STXBP2.32 A similar genetic disease, Griscelli syndrome subtype 2, is caused by a defect in the RAB27A gene.33 The proteins encoded by these genes are either cytolytic granule cargo (perforin) or elements of the secretory machinery required for lytic granule exocytosis (Munc13-4, Rab27a, syntaxin-11, and Munc18b). Our work demonstrated that Munc13-4 is a limiting factor for platelet granule secretion.34 Rab27a and b are important for platelet secretion.35 Recent studies of patients with FHL5 suggest that Munc18b is important for platelet function.36,37 These data imply that platelets use similar secretory machinery as cytotoxic T lymphocytes and NK cells. To explore this hypothesis and to further address whether Munc18b is required for platelet exocytosis, we analyzed platelets from 2 FHL5 patients with biallelic mutations and 1 heterozygous patient. We show that Munc18b is the major, platelet SM protein and is essential for dense and α-granule release. Western blotting and coimmunoprecipitation experiments indicate that syntaxin-11 is a cognate syntaxin for Munc18b in platelets and that Munc18b participates in complexes with other SNARE proteins (SNAP-23 and VAMP-8) and known regulators (Munc13-4 and Rab27a) of platelet secretion.
Methods
Antibodies and reagents
The goat anti-Munc18b antibody was obtained from Santa Cruz Biotechnology, Inc. The anti-Munc18a (clone131.1) was from Synaptic System. One anti–syntaxin-11 antibody (clone 32) was from BD Transduction Laboratory; a second anti–syntaxin-11 antibody, used for immunoprecipitations, is described in Ye et al.11 The anti–Munc13-4 antibody was generated by the use of fragment 703-975 a.a. as antigen. The antibodies to VAMP-8; SNAP-23; syntaxin-2, -4, and -7; -Munc18c; and -Rab27a have been described previously.7-9,34 The rabbit anti-Munc18b and anti–syntaxin-11 polyclonal antibodies were produced in our laboratory. FITC-labeled antihuman P-selectin was from BD Pharmingen. Alkaline phosphatase–conjugated anti-IgGs were from Sigma-Aldrich. The antiplatelet factor 4 (PF4) antibody for secretion assays was from R&D Systems.
Protein production and Western blotting analysis
Purified His6-Munc18a and His6-Munc18c were prepared from Escherichia coli extracts as described.28 His6-Munc18b was generated in Sf9 cells and purified by Ni2+-NTA chromatography. Western blotting of platelet samples and quantification was performed as described.34,38 To summarize, platelet proteins were separated on SDS-PAGE gels and electrophoretically transferred to Immobilon-P membranes (Millipore). The blots were incubated with different dilutions of primary and secondary antibodies. Antibody binding was detected by the use of enhanced chemifluorescence (ECF; GE Healthcare). Membranes were scanned with a Typhoon 9400 Imager and quantified with ImageQuant 5.2 software (GE Healthcare). Recombinant proteins were used to evaluate antibody specificity and to generate appropriate standard curves for quantification of the endogenous platelet proteins (Figure 1A-B).
Genotype of FHL5 patients
The genotypes of each patient were determined by standard sequencing methods and were as follows: patient 1 (P1), allele 1-389 T > C (L130S) and 1034 C > T (T345M), allele 2-1621 G > A (G541S); patient 2 (P2), allele 1-474-483 deletion GA, allele 2-1001 C > T (P334L); and patient 3 (P3), allele 1—no mutation, allele 2-1298 C > T (A433V). All procedures were approved by the institutional review boards at the Cincinnati Children's Hospital and at the University of Kentucky.
Platelet secretion analysis
Control and patient blood were collected using acid citrate dextrose (solution A) as an anticoagulant. Platelet secretion was measured as described.8,9 Washed platelets were labeled with 0.4 μCi/mL [3H]-5-HT (serotonin; Perkin Elmer Life Sciences) for 1 hour at 37°C. After being washing with HEPES/Tyrode buffer (10mM HEPES/NaOH, pH 7.4; 5.56mM glucose; 137mM NaCl; 12mM NaHCO3; 2.7mM KCl; 0.36mM KH2PO4; 1mM MgCl2) in the presence of 30 μg/mL apyrase, the platelets were resuspended in HEPES/Tyrode buffer. Platelet concentrations were adjusted to 2.5 × 108/mL, and a final concentration of 0.7mM CaCl2 was added before stimulation. For titration experiments, the indicated concentrations of thrombin (Chrono-Log) were added, and the reactions were stopped with a 2-fold excess of hirudin (Sigma-Aldrich). Supernatants and pellets were recovered after centrifugation at 16 430g for 1 minute, and the pellets were lysed with an equal volume of lysis buffer (PBS, pH 7.4, 1% Triton X-100) for 1 hour on ice. Equal volumes of supernatant and the pellet were assayed for the 3 granule cargo markers: [3H]-5–HT for dense granules, PF4 for α-granules, and β-hexosaminidase for lysosomes as described.7
Flow cytometry analysis
Human platelets (20 μL, 2 × 106/mL) were incubated with the indicated FITC-conjugated antibodies (5 μL) for 15 minutes. Platelets were stimulated with thrombin (0.1 U/mL) for 1 minute, and the reaction was stopped with a 2-fold excess of hirudin. Platelets were diluted 10-fold with HEPES-Tyrode buffer (pH 6.5). Fluorescent intensities of the platelets were measured with the FACScan flow cytometer and analyzed with CellQuest (BD Biosciences).
Platelet aggregation and ATP release
Human platelets were prepared as discussed previously, recalcified with 0.7mM CaCl2, placed into siliconized cuvettes, and stirred for 5 minutes at 37°C at 800 rpm. Luciferin-luciferase substrate was added to the platelet samples followed by the indicated agonists. ATP secretion was monitored using a Model 460VS Lumi-Dual aggregometer and traces were acquired using a Model 810 Aggro/Link interface with Aggro/Link rev. 5.1.5 software (Chrono-Log).
Ultrastructure analysis
Washed, human platelets were either kept resting or stimulated with 0.1 U/mL of thrombin for 3 minutes. The platelets were then processed for electron microscopy as described previously with slight modification.7,10 In summary, equal volumes of 0.1% glutaraldehyde in White saline39 were added to the platelet suspension for 15 minutes at 37°C. The platelets were centrifuged and incubated in ice-cold 3% glutaraldehyde in White saline at 4°C for 1 hour. After 3 washes, the platelets were incubated with 1% OsO4. Osmicated samples were washed twice and dehydrated with a series of ethanol solutions. The platelets were rinsed twice with propylene oxide and infiltrated overnight in a 1:1 mixture of propylene oxide and Spurr resin (10 g of vinyl cyclohexane dioxide, 6 g of DER epoxy resin, and 26 g of nonenyl succinic anhydride with final addition of 0.4 g of dimethylaminoethanol). After several washes in pure Spurr resin, samples were embedded in 150 μL of Spurr resin and polymerized in an incubator set at 60°C for 48 hours. Polymerized blocks were sectioned (70nM) and mounted on copper grids. After counterstaining them with uranyl acetate and lead citrate, we examined the samples using a Philips Tecnai 12 transmission electron microscope (FEI) and obtained images using Gatan Digitalmicrograph Version 1.83.842 software.
Immunoprecipitation of platelet SNAREs and regulatory proteins
Washed platelets were prepared for immunoprecipitations as previously described.40 For Munc18b immunoprecipitations, resting and stimulated platelets were incubated with 2× Lysis buffer (2% n-octyl β-D-glucopyranoside; 2mM EGTA; 2mM EDTA; 100mM HEPES; 150mM NaCl; 2mM Na3VO5 and protease inhibitor cocktail, pH 7.4) and kept on ice for 30 minutes. For the syntaxin-11 immunoprecipitation experiments, the 2× lysis buffer contained 2% Triton X-100 instead of 2% n-octyl β-D-glucopyranoside. Platelet lysates were centrifuged at 4°C to remove insoluble material. The nonspecific binding proteins were cleared by incubating platelet lysates with rabbit IgG (Sigma-Aldrich) and protein A-Sepharose beads for 30 minutes at 4°C. The cleared supernatant was then incubated with either anti-Munc18b, anti–syntaxin-11 rabbit polyclonal antibody, or control rabbit IgG for 2 hours at 4°C, followed by the addition of protein A-Sepharose beads for 2 hours at 4°C. The mixture was washed twice with 1× lysis buffer, and the bound proteins were eluted with SDS sample buffer and subjected to Western blotting with the indicated antibodies.
Results
Munc18b is the more abundant Munc18 isoform in normal human platelets
Platelets express Munc18a, Munc18b, and Munc18c.28 To determine the relative abundance of these 3 isoforms, recombinant proteins were first used to assess antibody specificity and then to generate standard curves for quantitative Western blotting using ECF. ECF coupled with the Typhoon Imager system offers a more linear response curve with a greater range than do other methods, for instance, ECF (Figure 1A). The specificity of the anti-Munc18a, -Munc18b, and -Munc18c antibodies used for this analysis was assessed by comparing their immunoreactivities to known amounts of the recombinant Munc18 proteins (Figure 1B). The anti-Munc18a rabbit monoclonal antibody shows a small degree of cross-reactivity to Munc18c. The goat anti-Munc18b antibody (used for quantification) was isoform-specific, whereas the rabbit anti-Munc18b did cross-react, slightly, with Munc18a. The anti-Munc18c antibody showed some cross-reactivity to Munc18a and even less to Munc18b. These limited cross-reactivities do affect the absolute accuracy of our analysis because they were not subtracted from our measurements; however, this has only a modest effect on their utility in ranking the per-platelet levels of the 3 Munc18 isoforms.
Munc18b is the major Munc18 isoform in platelets and is missing in platelets from FHL5/Munc18b/STXBP2patients. Recombinant Munc18a, b, and c were used to generate a standard curve for quantification (using ECF Western blotting) of each Munc18 isoforms in the indicated number of human platelets (A). Equal amounts of each Munc18 isoform were subjected to Western blotting with the antibodies used in this study to evaluate their cross-reactivity (B). Platelet extracts (1.0-2.0 × 107 platelets/lane) from control and FHL5 patients were probed by Western blotting with the indicated antibodies (C), and the ratio of patient to control levels of each protein was calculated and graphed (D).
Munc18b is the major Munc18 isoform in platelets and is missing in platelets from FHL5/Munc18b/STXBP2patients. Recombinant Munc18a, b, and c were used to generate a standard curve for quantification (using ECF Western blotting) of each Munc18 isoforms in the indicated number of human platelets (A). Equal amounts of each Munc18 isoform were subjected to Western blotting with the antibodies used in this study to evaluate their cross-reactivity (B). Platelet extracts (1.0-2.0 × 107 platelets/lane) from control and FHL5 patients were probed by Western blotting with the indicated antibodies (C), and the ratio of patient to control levels of each protein was calculated and graphed (D).
Acknowledging these limitations, we estimated that there were ∼ 2.5 ± 0.1 ng/5 × 107 of Munc18a (∼ 450 molecules per platelet, n = 6), 56.1 ± 3.71 ng/5 × 107 of Munc18b (∼ 10 240 molecules per platelet, n = 6), and 27.8 ± 0.82 ng/5 × 107 of Munc18c (∼ 5070 molecules per platelet, n = 6). On the basis of this analysis, Munc18b is ∼ 2-fold more abundant than Munc18c and ∼ 25-fold more abundant than Munc18a. Despite the limited uncertainty introduced by the antibody cross-reactivities, these data clearly show that Munc18b is the more abundant Munc18 isoform in human platelets.
Characterization of Munc18s and SNARE proteins in FHL5 platelets
Investigators who have used inhibitory peptides or antibodies have suggested roles for Munc18a and Munc18c in platelets27,29,41 ; however, their conclusions were not supported by studies with heterozygous, Munc18c knockout mice, whose platelets did not have a secretion defect.29 FHL5 is linked to a defect in the Munc18b/STXBP2 gene, which provides a unique opportunity to examine Munc18b's role in platelet function. In Figure 1C and D, Munc18b was undetectable in platelets from the 2 biallelic patients (P1 and P2). The heterozygous patient (P3) showed a ∼ 30% reduction. Munc18a increased in both biallelic samples, but this increase was apparently not sufficient to compensate for the loss of Munc18b (Figures 2 and 3). Munc18c levels were unchanged (Figure 1C-D). These data indicate that the mutations in the 2 biallelic patients (P1 and P2) cause the production of an unstable Munc18b protein, which was not detectible in platelets. The mutation in the heterozygous patient (P3) had less effect on Munc18b protein levels.
Platelets from FHL5/Munc18b/STXBP2 patients have a secretion defect. Platelet-rich plasma was generated by centrifugation and labeled with [3H]-serotonin. After washing, platelets were stimulated with increasing concentrations of thrombin for 1 minute, and then the release of [3H]-serotonin (Dense), PF4 (Alpha), and β-hexosaminidase (Lysosomes) were measured as described under “Platelet secretion analysis” (A and B). (A) Release profiles for biallelic patient 1 (open symbols) and a normal control (closed symbols). Patient 2 showed a similar profile. (B) Release profiles for heterozygous patient 3 (open symbols) and a normal control (closed symbols). [3H]-serotonin, PF4, and β-hexosaminidase in the releasate and remaining in the platelets were measured, and a percent secretion was calculated for each treatment condition. The data are the averages of triplicate measurements with the SD indicated.
Platelets from FHL5/Munc18b/STXBP2 patients have a secretion defect. Platelet-rich plasma was generated by centrifugation and labeled with [3H]-serotonin. After washing, platelets were stimulated with increasing concentrations of thrombin for 1 minute, and then the release of [3H]-serotonin (Dense), PF4 (Alpha), and β-hexosaminidase (Lysosomes) were measured as described under “Platelet secretion analysis” (A and B). (A) Release profiles for biallelic patient 1 (open symbols) and a normal control (closed symbols). Patient 2 showed a similar profile. (B) Release profiles for heterozygous patient 3 (open symbols) and a normal control (closed symbols). [3H]-serotonin, PF4, and β-hexosaminidase in the releasate and remaining in the platelets were measured, and a percent secretion was calculated for each treatment condition. The data are the averages of triplicate measurements with the SD indicated.
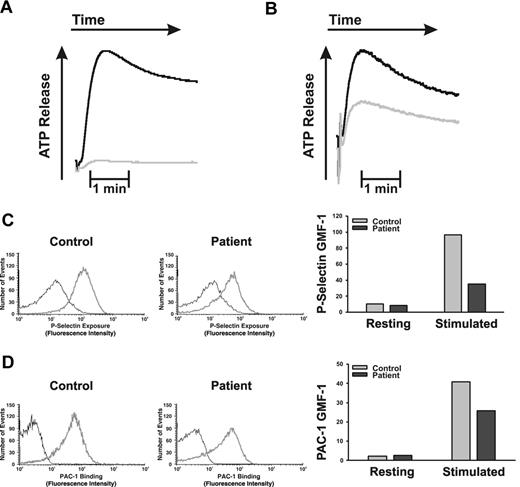
Platelets from FHL5/Munc18b/STXBP2 patients have a defect in ADP/ATP release and P-selectin exposure. Washed platelets were stimulated with thrombin (0.1 U/mL), and the release of ADP/ATP from dense granules was measured in a lumi-aggregometer as described previously.34 (A) ATP release profile from a normal control (black line) and biallelic patient 1 (gray line). (B) Release profiles for a normal control (black line) and a heterozygous patient 3 (gray line). (C) Resting (black line) thrombin-stimulated (gray line) platelets from control, or biallelic, patient 1 were incubated with FITC-conjugated anti–P-selectin antibodies for 1 minute at room temperature. The reactions were stopped with hirudin, and the stained platelets were analyzed by flow cytometry. A similar experiment was repeated with PAC-1 antibodies (D).
Platelets from FHL5/Munc18b/STXBP2 patients have a defect in ADP/ATP release and P-selectin exposure. Washed platelets were stimulated with thrombin (0.1 U/mL), and the release of ADP/ATP from dense granules was measured in a lumi-aggregometer as described previously.34 (A) ATP release profile from a normal control (black line) and biallelic patient 1 (gray line). (B) Release profiles for a normal control (black line) and a heterozygous patient 3 (gray line). (C) Resting (black line) thrombin-stimulated (gray line) platelets from control, or biallelic, patient 1 were incubated with FITC-conjugated anti–P-selectin antibodies for 1 minute at room temperature. The reactions were stopped with hirudin, and the stained platelets were analyzed by flow cytometry. A similar experiment was repeated with PAC-1 antibodies (D).
Loss of a Munc18 isoform often correlates with the loss of a specific syntaxin isoform and vice versa, consistent with the syntaxin chaperone function of Munc18s.42 In Figure 1C-D, the loss of Munc18b caused a striking decrease in syntaxin-11 with limited effects on syntaxin-4 (syntaxin-2, -7, and SNAP-23 were unchanged in biallelic patient [P1], data not shown). This finding is consistent with other studies of T cells from FHL5 patients.20,21 VAMP-8, Munc13-4 (FHL3), and Rab27a levels were unaffected. Although not proof of a direct interaction, these data are consistent with Munc18b acting as a chaperone for syntaxin-11 in platelets.
Thrombin-stimulated secretion is defective in FHL5 platelets
To address the functional ramifications of the loss of Munc18b, dose–response, secretion assays were performed to examine platelet exocytosis from biallelic and heterozygous FHL5 patients (Figure 2A-B, respectively). In Figure 2A, increasing concentrations of thrombin were used to stimulate platelets, and the release from dense granules, α-granules, and lysosomes was measured. In control platelets (dark circles), there was a dose-dependent increase in serotonin, PF4, and β-hexosaminidase secretion that reached a maximum between 0.3 and 0.5 U/mL thrombin. Platelets from the biallelic patient (P1, open circles) exhibited a severe defect in release from both dense and α-granules, even at the greatest concentration of thrombin used (0.5 U/mL). β-hexosaminidase release also was affected compared with the control; however, this defect seemed to be overcome when greater concentrations of thrombin were used. Similar effects were seen in the second biallelic patient (P2, data not shown).
The heterozygous FHL5 patient (P3) showed a partial secretion defect (Figure 2B). Serotonin and PF4 release from P3's platelets were attenuated and did not reach the same extent as seen for the control platelets. Lysosome release was largely unaffected, which indicates that the reduction in Munc18b levels observed in the platelets from P3 does affect secretion efficacy. It is of note that there was little if any loss of syntaxin-11 in this patient's platelets (Figure 1C-D). This argues that the secretory defect in the heterozygous patient is largely because of a loss of Munc18b but not syntaxin-11. Furthermore, the fact that a secretion defect is noted in a heterozygous patient suggests that Munc18b could be a limiting component of the secretory machinery. A similar haploinsufficiency was noted in heterozygous Unc13-4Jinx mouse platelets, where it was demonstrated that Munc13-4 was a limiting component of the secretory machinery.34
As 2 additional measures of platelet secretion, we examined the release of ADP/ATP (using the luciferin/luciferase assay in a lumi-aggregometer) and P-selectin exposure by (FACS). ADP/ATP release is a metric of dense granule release, and P-selectin exposure is a metric of α-granule secretion. In Figure 3A, ADP/ATP was almost completely absent from platelets from the biallelic patient, P1, and P-selectin exposure was greatly affected (64% reduction; Figure 3C). Interestingly, αIIbβ3 integrin activation as measured by PAC-1 antibody binding was slightly affected in the platelets from patient P1 (reduced by 37%, Figure 3D) perhaps because of the lack of ADP/ATP release (reviewed in Nieswandt et al43 ). As seen in Figure 2B, the release of ADP/ATP from dense granules of the heterozygous patient's platelets (Figure 3B) was attenuated but not totally deficient. These data are consistent with the secretion defects seen in Figure 2 and further confirm that Munc18b plays a role in dense and α-granule cargo release.
Ultrastructure of FHL5 platelets
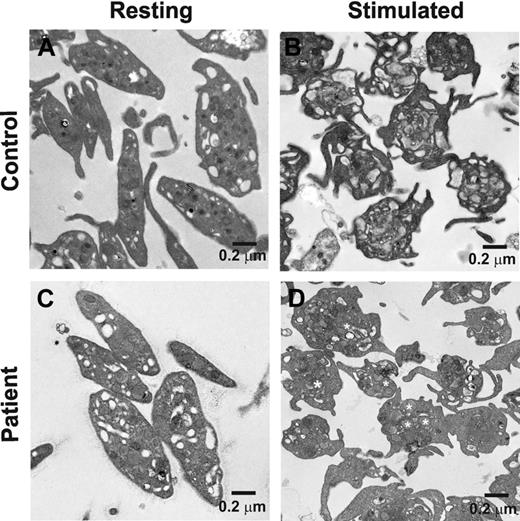
Platelet activation leads to actin cytoskeleton rearrangements that play a role in platelet secretion44,45 ; therefore, it is possible that a loss of Munc18b affects the platelets cytoskeleton rearrangements, thus indirectly affecting secretion. This is especially true given the recent studies of how Munc18-1 affects cortical F-actin in chromaffin cells.46 To determine whether activation-dependent cytoskeletal changes were affected, both resting and thrombin-stimulated platelets from control and a biallelic FHL5 patient (P1) were examined by electron microscopy (Figure 4). Resting platelets had a normal discoid shape with similar distributions of identifiable granules, vacuoles, and mitochondria. There were no overt differences in the morphology of normal and FHL5 patient's platelets. These results are consistent with our measurements of total granule cargo levels ([3H]-serotonin loaded, PF4, or β-hexosaminidase used to calculate percent secretion); there were no significant differences between normal controls and any of the FHL5 patients (data not shown).
The ultrastructure of resting and stimulated platelets from a FHL5/Munc18b/STXBP2 patient. Platelets from a biallelic patient (P1; A and C) and normal control (B and D) were prepared as described under “Ultrastructure analysis.” (A and B) Platelets under resting conditions. Thrombin (final concentration 0.1 U/mL) was added to samples shown in panels C and D. After 2 minutes at room temperature, samples were fixed and analyzed by transmission electron microscopy. (D) Some α-granules are marked with white asterisks. The scale bar is indicated in all images.
The ultrastructure of resting and stimulated platelets from a FHL5/Munc18b/STXBP2 patient. Platelets from a biallelic patient (P1; A and C) and normal control (B and D) were prepared as described under “Ultrastructure analysis.” (A and B) Platelets under resting conditions. Thrombin (final concentration 0.1 U/mL) was added to samples shown in panels C and D. After 2 minutes at room temperature, samples were fixed and analyzed by transmission electron microscopy. (D) Some α-granules are marked with white asterisks. The scale bar is indicated in all images.
On activation, both normal and FHL5 platelets showed the same changes in shape, sending out filipodia consistent with activation-dependent alterations in the actin cytoskeleton. After activation, the normal controls showed few identifiable granules, consistent with cargo release. However, the activated FHL5 platelets showed clearly visible granules. This implies that the cytoskeletal rearrangements had centralized the granules but that they had failed to fuse and release their contents. Such a phenotype is consistent with the secretion defect described previously and has been noted in previous studies of VAMP-8−/−, Unc13-4Jinx, and FHL4 platelets in which secretion is clearly defective (Ren et al7,34 and Ye et al11 ). These data indicate that the loss of Munc18b does not affect the activation-dependent actin cytoskeletal rearrangements but does affect granule-plasma membrane fusion.
Munc18b associates with SNAREs and SNARE regulators
Previous work demonstrated that platelet secretion requires the v-SNARE VAMP-8 and the t-SNAREs syntaxin-11 and SNAP-23.7-11 These proteins are proposed to form the trans-membrane complex that is required for membrane fusion and granule cargo release.6 The data presented here suggest that Munc18b and syntaxin-11 levels are partially codependent (Figure 1C-D), consistent with Munc18b serving as a syntaxin-11 chaperone in platelets. If Munc18b is a syntaxin-11 regulator and thus part of the platelet secretory machinery, one might expect that Munc18b could form a complex with at least the t-SNARE complex: syntaxin-11 and SNAP-23, if not the whole SNARE complex: VAMP-8, SNAP-23, and syntaxin-11.
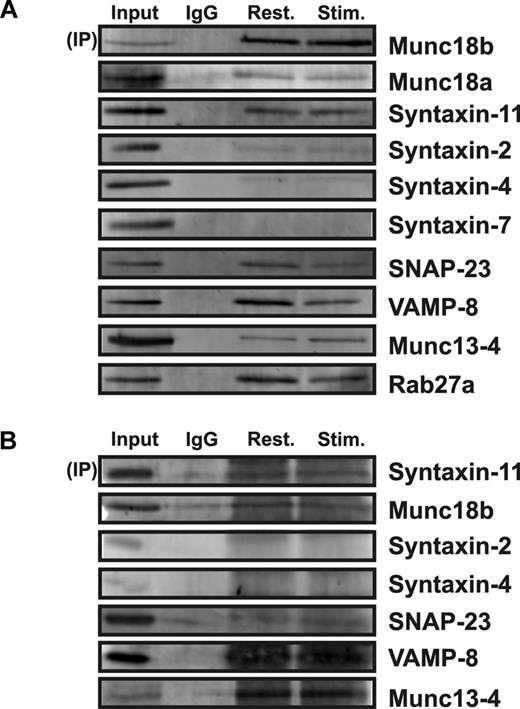
To investigate this, we used coimmunoprecipitation and Western blotting to determine which elements of the platelet secretory machinery associate with Munc18b. In Figure 5A, immunoprecipitation of Munc18b brought down syntaxin-11, SNAP-23, and VAMP-8. There was no syntaxin-4 or -7 detectible. The limited syntaxin-2 present could be the result of cross-reactivity of the blotting syntaxin-2 antibody for syntaxin-11.11 Alternatively, because the rabbit anti-Munc18b used for these experiments has a slight cross-reactivity for Munc18a, it could have coprecipitated Munc18b/syntaxin-2. Despite these caveats, it appears that the major cognate syntaxin for Munc18b in platelets is syntaxin-11. Under these precipitation conditions, there were no obvious differences in the complexes recovered from resting or stimulated platelets.
Munc18b associates with syntaxin-11 and with other SNARE proteins. Extracts from resting or stimulated platelets were prepared with 2% n-octyl β-glucopyranoside for immunoprecipitation (IP) with rabbit anti-Munc18b antibodies (A) or 0.5% Triton-X100 for IP with rabbit anti–syntaxin-11 antibodies (B). IgG was used as a specificity control (A and B) and the immune-complexes were recovered with protein A-Sepharose beads. The bound complexes were then analyzed by Western blotting by the use of antibodies to the indicated proteins.
Munc18b associates with syntaxin-11 and with other SNARE proteins. Extracts from resting or stimulated platelets were prepared with 2% n-octyl β-glucopyranoside for immunoprecipitation (IP) with rabbit anti-Munc18b antibodies (A) or 0.5% Triton-X100 for IP with rabbit anti–syntaxin-11 antibodies (B). IgG was used as a specificity control (A and B) and the immune-complexes were recovered with protein A-Sepharose beads. The bound complexes were then analyzed by Western blotting by the use of antibodies to the indicated proteins.
Similar results were obtained when a rabbit anti–syntaxin-11 polyclonal antibody was used to precipitate complexes. Munc18b, as well as VAMP-8 and SNAP-23, was detected in the immunoprecipitate. This finding is consistent with a previous report showing that Munc18b associates with SNARE complexes purified from platelet extracts using a general affinity technique involving NSF binding.28 These data show that Munc18b associates with syntaxin-11 and that it does interact with other components of the core fusion machinery. To expand this observation, we also probed for other elements of the secretion machinery that are known to be required. Interestingly, both Rab27a and Munc13-4 (a Rab27 effector47 ) were found to coprecipitate with Munc18b. Although this finding might argue that a larger “fusion” complex is present in platelets, further experiments will be required to define the interactions implied by these findings.
Discussion
The present study shows that Munc18b is more abundant than the other Munc18 isoforms present in platelets. On the basis of previous studies of VAMP-8,7 syntaxin-11,11 SNAP-23,8-10,41 and Munc13-4,34 it appears that the most abundant isoform of a given secretory protein family is the most functionally relevant in platelets. This conclusion is supported by the platelet phenotype of patients with FHL5, a defect in the Munc18b gene. In 2 biallelic patients and 1 heterozygous patient, there was a clear loss of Munc18b protein. The decrease in platelet Munc18b suggests that the point mutations (L130S, P334L, T345M, and G542S) destabilize Munc18b, leading to its degradation in platelets. Coincident with the loss of Munc18b, there were clear defects in release from dense (serotonin and ADP/ATP) and α-granules (PF4 and P-selectin exposure).
These data are consistent with the FACS-based analysis of platelets from FHL5 patients reported by Sandrock et al.37 The attenuated defect seen in the heterozygous patient argues that Munc18b might be a limiting component of the secretory machinery in platelets. We have seen similar results in heterozygous Unc13dJinx mice that have reduced levels of Munc13-4.34 The FHL5 patients' platelets showed no overt difference in morphology or granule cargo content, suggesting that granule biosynthesis was normal. The phenotype of the stimulated platelets indicates that cytoskeletal changes do occur normally and that the loss of Munc18b truly affects fusion because there are intact granules in the stimulated platelets from the FHL5 patient. The loss of Munc18b caused a decrease in syntaxin-11, which is consistent with earlier studies of cells from FHL5 patients.20,21
These data suggest that Munc18b/syntaxin-11 is a functionally relevant pair in both lymphocytes and platelets. We confirmed this via immunoprecipiation experiments, which revealed that the 2 proteins do interact in a complex. This concept is further supported by the secretion defects noted in our analysis of platelets from a FHL4 patient who lacks syntaxin-11.11 Finally, the fact that Munc18b was found associated with other core elements of the SNARE fusion machinery (VAMP-8/SNAP-23/syntaxin-11) and its regulators (Munc13-4/Rab27) further establishes it as a key element of the platelet secretory machinery.
Contrary to the data presented here, previous studies, using potential inhibitors in permeabilized platelets, had established the importance of Munc18s in platelet secretion. Our own work with peptides, thought to affect the interactions of Munc18s with other regulators such as Rabs, showed that Munc18s were needed.28 However, the sequences were highly conserved, and thus we could not establish which Munc18 isoform was required for calcium-stimulated release. Other studies used a cocktail of peptides and a monoclonal antibody to disrupt Munc18c/syntaxin-4 complexes. These reagents enhanced release from permeabilized platelets.27,41 Such results supported an original hypothesis that Munc18s were negative regulators of syntaxins, preventing their interactions with other SNAREs, and thus were “brakes” on secretion. However, in recent years, our understanding of Munc18 function has expanded. It is now thought that some Munc18s stay associated with SNAREs throughout the secretion process and actually contribute to membrane fusion.11 The data presented here and those reported by Schraw et al28 argue that, in platelets, Munc18b stays associated with the complete SNARE complex not just the t-SNARE heterodimer (syntaxin/SNAP-23).
Other initial observations also were clarified by the data presented here. Loss of Munc18c (∼ 30% reduction) did not adversely affect secretion from mouse platelets.29 This was initially thought to be because the heterozygous mouse platelets did not have a large enough deficit of Munc18c to affect platelet secretion. Given the phenotype of the heterozygous patient's platelets reported here, one would expect that if Munc18c was critical and limiting, as Munc18b appears to be, even loss of a fraction of the protein should affect platelet secretion. Finally, the studies in the accompanying paper by Ye et al failed to show a role for syntaxin-4, the cognate t-SNARE for Munc18c, in platelet secretion.11 Thus, although we cannot explain some of the original results, the data presented here clearly establish that Munc18b is required for platelet secretion.
FHL is a generally fatal genetic disease that requires rapid diagnosis and treatment. Hematopoietic stem cell transplantation is required for a favorable prognosis. Five FHL types are known, and the genes for 4 (FHL2/perforin, FHL3/Munc13-4, FHL4/syntaxin-11, and FHL5/Munc18b/STXBP2) have been identified.48,49 With the present study, we have now characterized the secretion phenotype of platelets lacking FHL3/Munc13-4,34 FHL4/syntaxin-11,11 and FHL5/Munc18b and in every case there has been a defect in secretion from at least dense and α-granules. FHL can present with various symptoms, and its diagnosis can be difficult, given the young age of the patients. As argued recently by Pagel et al, our data support the addition of platelet function assays to the routine examination of FHL patients to establish whether such assays can be used as a diagnostic tool.50 It should be noted that bleeding histories may be too variable to be a sufficient diagnostic criteria. Platelet function assays, such as lumi-aggregometry or FACS-based tests, are better metrics for the secretion defects that are associated with these 3 types of FHL.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Mary Gail Engle and Jim Begley of the Chandler Medical Center Imaging Facility. Flow cytometry was carried out with the assistance of Greg Bauman at the University of Kentucky Flow Cytometry and Cell Sorting Facility, which is supported by the Office of the Vice President for Research, the Markey Cancer Center, and a grant from the National Institutes of Health Share Instrument Program (S10 RR026827). They thank the Kentucky Blood Center for their help. They are also indebted to the members of the Whiteheart laboratory for their editorial comments during the preparation of the manuscript.
This work is supported by National Institutes of Health grants (HL56652 and HL082193) to S.W.W. and (AI079759 and AI076746) to A.H.F. and a grant from the Histiocytosis Association of America to A.H.F.
National Institutes of Health
Authorship
Contribution: R.A.H., Q.R., S.Y., and Z.A.K. performed the experiments and prepared the figures; A.H.F. recruited the patients; S.W.W. analyzed the data; and R.A.H., Q.R., and S.W.W. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Q.R. is the Department of Protein Chemistry, Beijing Novo Nordisk Pharmaceuticals Science & Technology Co. Ltd, Changping District, Beijing, China.
Correspondence: Dr Sidney W. Whiteheart, PhD, Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, 741 South Limestone, Biomedical Biological Sciences Research Building, Lexington, KY 40536-0509; e-mail: whitehe@uky.edu.


![Figure 2. Platelets from FHL5/Munc18b/STXBP2 patients have a secretion defect. Platelet-rich plasma was generated by centrifugation and labeled with [3H]-serotonin. After washing, platelets were stimulated with increasing concentrations of thrombin for 1 minute, and then the release of [3H]-serotonin (Dense), PF4 (Alpha), and β-hexosaminidase (Lysosomes) were measured as described under “Platelet secretion analysis” (A and B). (A) Release profiles for biallelic patient 1 (open symbols) and a normal control (closed symbols). Patient 2 showed a similar profile. (B) Release profiles for heterozygous patient 3 (open symbols) and a normal control (closed symbols). [3H]-serotonin, PF4, and β-hexosaminidase in the releasate and remaining in the platelets were measured, and a percent secretion was calculated for each treatment condition. The data are the averages of triplicate measurements with the SD indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/12/10.1182_blood-2012-05-430629/4/m_zh89991295630002.jpeg?Expires=1767801946&Signature=Ly8Q9GdKPSJ2x3eNbB43MczN21KmqokRBcvBB2cNYxtLdOiZbkC-6Gl7NBK2SZf3MqB5~oncfNivtAHYoVcSL0nBq9BILgrVZvHX2SfdYqMG8HMvNSVA0GqxvSnmf9iOHb9JiRC01qHdj-c7zoloVsxIidj7Oti5K6076kcqjHg8Rfa27wO0L1jB7cCdrb-KUzk4TxRHdYUjm55eh0l7UO0ulOAGkP1uPhELnAKyhKthW~jFHni08o4rmJ-dbBW5kSfTwX5RCuDWORmQCBdwsJz1K36QaGxTIhgE3CuT8RNubceIzZR3bcCdxNAnMDRT~aY3L-0FSu6-uZd81aDN0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal