In this issue of Blood, Jacobsohn et al report that 2 simple and reproducible assessment tools, the National Institutes of Health (NIH) composite skin score and the Lee skin symptom scale, are powerful predictors of severity and prognosis of skin GVHD.1
Allogeneic transplantation was established as a curative procedure for hematologic malignancies in the early 1970s. Soon thereafter, a novel syndrome of chronic GVHD (cGVHD) was described with pleotropic clinical manifestations, multiple organ involvement, and a special predilection for the skin.2 From its earliest descriptions it was determined that cGVHD was a disease with a high fatality rate and that it frequently affected patients' quality of life. Based on detailed analysis of 20 patients, limited and extensive cGVHD were proposed.3 Extensive cGVHD diminished function, quality of life, and life expectancy. But others found an inverse correlation between the occurrence of cGVHD and risk for disease recurrence in leukemia.4 Ever since, observations have been reported associating mild cGVHD with improved long-term survival, while severe cGVHD is almost invariably associated with worse outcomes. Forty years later, definitions and classifications of cGVHD have been revised, but its management remains disappointing.5 First-line treatment relies on steroids or steroids with calcineurin inhibitors.6 More aggressive interventions such as the addition of mycophenolate mofetil do not improve long-term survival.7 What constitutes effective second-line treatment also remains to be determined. The results of promising phase 2 treatments have not often been confirmed in phase 3 treatments. Extracorporeal photopheresis, although widely considered beneficial and used frequently for advanced skin GVHD, did not achieve its primary end point when tested in a randomized study.8 Progress has been hindered by our limited understanding of the biology of cGVHD. But equally important is the lack of robust, reproducible, and validated methods for assessing the severity of cGVHD and its response to treatment.
Several instruments—some of considerable complexity—have been proposed for assessment of skin GVHD, the most frequent target organ. Now, Jacobsohn et al report a prospective multicenter validation of these tools. For this purpose they followed 458 transplant patients and assessed severity of skin GVHD with various instruments. They compared the results of the instrument-generated scores with physician and patient assessment of improvement over time.
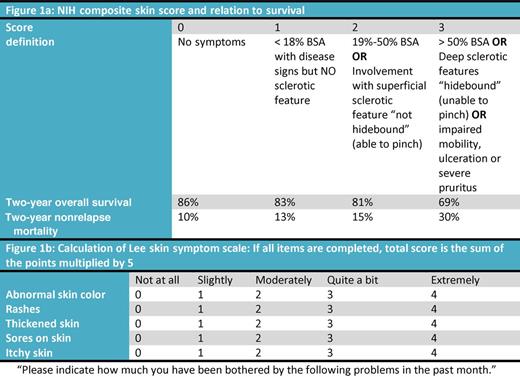
Assessment of the extent and severity of skin involvement using the NIH composite skin score (see figure panel A)5 correlated well with severity of symptoms. By contrast, more complicated assessments correlated poorly with patient or physician perception. The same NIH composite skin score also correlated with survival. Worsening in NIH skin score at 6 months was associated with higher mortality and higher nonrelapse mortality compared with stable NIH skin score. The latter observation suggests that the NIH composite skin score could serve as a short-term surrogate end point that predicts for long-term success or failure of novel drug therapies.
Another straightforward assessment tool completed by patients, the skin subscale of the Lee symptom scale (see figure panel B)9 also correlated well with physician and patient perception of improvement in cGVHD. A skin score of more than 15 correlated with worse survival, whereas improvement on the symptom scale was associated with improvement in survival.
This work provides validation of the proposals of the 2005 Chronic GVHD Consensus conference, and generates robust and reproducible tools that can be used for the assessment of novel therapies.10 A better delineation of risk categories of skin GVHD also constitutes a pivotal step toward generating better understanding of the disease biology.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal