Abstract
The allantois is the embryonic precursor of the umbilical cord in mammals and is one of several embryonic regions, including the yolk sac and dorsal aorta, that undergoes vasculogenesis, the de novo formation of blood vessels. Despite its importance in establishing the chorioallantoic placenta and umbilical circulation, the allantois frequently is overlooked in embryologic studies. Nonetheless, recent studies demonstrate that vasculogenesis, vascular remodeling, and angiogenesis are essential allantois functions in the establishment of the chorioallantoic placenta. Here, we review blood vessel formation in the murine allantois, highlighting the expression of genes and involvement of pathways common to vasculogenesis or angiogenesis in other parts of the embryo. We discuss experimental techniques available for manipulation of the allantois that are unavailable for yolk sac or dorsal aorta, and review how this system has been used as a model system to discover new genes and mechanisms involved in vessel formation. Finally, we discuss the potential of the allantois as a model system to provide insights into disease and therapeutics.
Introduction
Developmental origin and vascular development of the allantois
The allantois of the mouse emerges from the posterior end of the embryo at the neural plate stage of development as a bud of mesoderm derived from the primitive streak.1,2 As development proceeds, the allantois grows toward the chorion through the exocoelomic cavity. Its distal end undergoes a process of cavitation, whereby the extracellular spaces increase in volume and accumulate hyaluronic acid (HA).3 Contact and fusion between the allantois and the chorion occurs at the 4-6 somite pair (s) stage, followed by spreading of the allantois on the surface of the chorion to form the chorio-allantoic plate. The chorion forms the placental villi, and the vasculature of the allantois grows into these villi to form the labyrinthine layer of the placenta (Figure 1).4
Diagrammatic representation of the developing mouse embryo from the neural plate (allantoic bud) stage to the 22-26 somite (s) stage highlighting the development of the allantois and its vasculature. The age in embryonic days (E), and the stages of development are indicated across the top, as are the developmental processes taking place in the allantois. The allantois first appears as a mesodermal bud emerging from the posterior end of the primitive streak. The bud grows and expands across the exocoelomic cavity and fuses with the chorion to form the chorioallantoic placenta. The processes in allantois vessel development at each stage are indicated across the bottom of the figure. ECs differentiate and migrate to form a primary vascular plexus. The plexus connects with the dorsal aortae of the embryo before chorio-allantoic fusion. The primary plexus remodels to form the central vessel; later, the umbilical artery and the umbilical vein form at E9.5. After chorio-allantoic fusion, the allantoic vessels invade the chroion and form the fetal vascular component of the labyrinthine layer of the placenta. C indicates allantois core; CAP, chorio-allantoic plate; DA, dorsal aorta; epc, ectoplacental cone; exc, exocoelomic cavity; lab, placental labyrinth; m, mesothelium; NP, neural plate; UA, umbilical artery; UV, umbilical vein; and ys, yolk sac.
Diagrammatic representation of the developing mouse embryo from the neural plate (allantoic bud) stage to the 22-26 somite (s) stage highlighting the development of the allantois and its vasculature. The age in embryonic days (E), and the stages of development are indicated across the top, as are the developmental processes taking place in the allantois. The allantois first appears as a mesodermal bud emerging from the posterior end of the primitive streak. The bud grows and expands across the exocoelomic cavity and fuses with the chorion to form the chorioallantoic placenta. The processes in allantois vessel development at each stage are indicated across the bottom of the figure. ECs differentiate and migrate to form a primary vascular plexus. The plexus connects with the dorsal aortae of the embryo before chorio-allantoic fusion. The primary plexus remodels to form the central vessel; later, the umbilical artery and the umbilical vein form at E9.5. After chorio-allantoic fusion, the allantoic vessels invade the chroion and form the fetal vascular component of the labyrinthine layer of the placenta. C indicates allantois core; CAP, chorio-allantoic plate; DA, dorsal aorta; epc, ectoplacental cone; exc, exocoelomic cavity; lab, placental labyrinth; m, mesothelium; NP, neural plate; UA, umbilical artery; UV, umbilical vein; and ys, yolk sac.
The growing allantois, which consists of an outer layer of mesothelium enveloping a mesenchymal core, increases in size both by cell proliferation and by addition of cells from the primitive streak.1 De novo endothelial cell (EC) differentiation starts in the distal allantois as early as the headfold (HF) stage and is characterized by expression of the Flk1 (also known as Vegfr-2), Pecam, and VE-Cadherin (VE-Cad).5 Starting at the 1s stage, the ECs coalesce in a distal-to-proximal direction to form vessels in a process termed vasculogenesis. These vessels form the primary vascular plexus, evident at the 4s stage, which joins the embryonic and yolk sac circulation at the base of the allantois.1 The primary vascular plexus remodels to form the umbilical artery and vein,1 which then invade the chorion by sprouting angiogenesis (Figure 1; for a review of vasculogenesis versus angiogenesis, see Rossant and Howard6 ). It is still an open question whether the allantois contributes to the definitive hematopoietic cell lineage. Cells of the allantois do express markers of hematopoietic cell progenitors and can differentiate to form cells of the definitive erythroid and myeloid lineages in vitro,7,8 but whether this occurs in vivo is unknown.
Unlike the allantois, where ECs migrate through the extracellular matrix (ECM) of a mesenchymal structure to form the primary vascular plexus, the yolk sac vascular plexus forms within a bilaminar epithelial structure, between the endoderm and mesothelium. Clusters of cells within the mesoderm form the yolk sac blood islands, which contain hemangioblasts, common precursors of ECs, hematopoietic cells, and smooth muscle cells (SMCs; Figure 1).9 By embryonic day (E) 8.5, the blood islands contain a single layer of ECs that surround the hematopoietic cells. The ECs then connect to form a continuous primary vascular plexus, which is remodeled into large and small vitelline vessels.10 In the embryo, vasculogenesis within the lateral plate mesoderm is dependent on endodermal signaling, similar to the yolk sac, but unlike the allantois. Angioblasts, the precursors of ECs, appear in the lateral plate mesoderm ventral to the somites and migrate medially to fuse together forming the paired dorsal aortae (Figure 1). The dorsal aortae connect anteriorly with the aortic arteries exiting the primitive heart tube and fuse posteriorly to form a single dorsal aorta in the midline at the level of the forelimbs.11 In the lateral plate mesoderm, hematopoiesis takes place in the dorsal aorta from the hemogenic endothelium lining the blood vessels.12
Although the genetic control of vasculogenesis and angiogenesis has been well reviewed in the embryo and yolk sac,6,13-18 similar attention has not been given to the allantois, despite the importance of allantois vasculogenesis, vascular remodeling, and angiogenesis in the establishment of the chorioallantoic placenta. In this review, we compile information for the allantois about genes known to be important for differentiation of vascular components and vascular network formation. In many cases, information for the allantois vasculature is lagging behind that for vasculogenesis or angiogenesis in other parts of the embryo, and thus, we highlight areas in which there are still gaps in our knowledge. We discuss methods for the culture of allantoises ex vivo, an experimental system that is unavailable for yolk sac or dorsal aorta, and review how this has been used as a model system to investigate vessel formation in both normal and mutant allantoises (Table 1),2,5,7,19-60 with the potential to provide insights into disease and therapeutics.
Vasculature-related genes expressed in the allantois or placenta and their corresponding phenotypes in the mutant allantois or placenta
| Category/gene name . | Expression in the allantois or placenta . | Ref. . | Phenotype in allantois or placenta . | Ref. . | |
|---|---|---|---|---|---|
| Age . | Area of expression . | ||||
| Vegf signaling | |||||
| Vegf | E8.5, E12.5 | Mt, labyrinthine stroma | 19,20 | Degenerating endothelium in placenta | 21 |
| Flk1 | E7.5, E8.75, E12.5 | EC, placental vasculature | 5,19,20 | Lack of mature ECs in the allantois | 22 |
| Flt1 | E8.75, E12.5 | EC, placental vasculature | 5,19,20 | Nd | |
| EC | |||||
| Pecam | E8.0 onwards | EC | 5 | None (viable) | 23 |
| VE-Cad | E8.0 onwards | EC | 5 | No allantois plexus | 24-27 |
| Hematopoietic | |||||
| Tal1 | E7.0-8.0 | EPC | 5 | Absence of blood | 28 |
| Runx1 | E8.0-8.5 | Hematopoietic precursors | 7 | Nd | |
| SMC | |||||
| SM22α | E8.25 | SMC | None (viable) | 29 | |
| αSMA | E8.25 | SMC | Nd | ||
| ECM | |||||
| Fn | E8.25 | Mes* | 30 | Nd | |
| Itgα5 | E8.25 | Mes | 31 | Nd | |
| Collagen1α (I) | E8.25 | Mes | 31 | Nd | |
| Has2 | E8.25 | Mes | 31 | Nd | |
| Vcan | E8.25 | Mes | 31 | Nd | |
| Adamts9 | E7.5 | Mes | 32 | Nd | |
| Remodeling of vascular plexus | |||||
| Nrp1 | Nd | Nd | Nd | ||
| Nrp2 | Nd | Nd | Nd | ||
| Tie1 | E8.75, E12.5 | EC, placental vasculature | 19,20 | Nd | |
| Tie2 | E8.75, E12.5 | EC, placental vasculature | 19,20 | Nd | |
| Ang1 | Nd | Nd | Lack of complexity in umbilical vessels | 33 | |
| Ang2 | Nd | Nd | Nd | ||
| Tgfβ | E7.5-8.5 | Nd | 34 | Defective chorio-allantoic fusion; lack of Flk1 expression in allantois | 35 |
| Alk1 | Nd | Nd | Nd | ||
| Alk5 | Nd | EC and SMC of placenta | 36 | Defective sprouting of allantois into placenta; lack of SMCs around allantoic vessels | 36 |
| Edd | Nd | Nd | ECs fail to form plexus, lack of chorio-allantoic fusion | 37 | |
| Lpp3 | E8.0-E10.5 | Mes | 38 | ECs fail to form plexus, lack of chorio-allantoic fusion | 38 |
| Artery versus vein | |||||
| ephrinB2 | E9.5 | Vascular plexus, UA | 39 | None | 40 |
| EphrinB4 | E9.0 | EC/UV | 39 | Nd | |
| Notch2 | E8.25 | Mes | 31,41 | Nd | |
| Dll4 | E8.25 | Arterial EC | 31 | Degenerating vasculature in placenta | 42 |
| Jag1 | E8.25 | Mes | 31 | Nd | |
| Hey1 Hey2 | E8.5, E9.0 | Mes | 43 | Vasculature in CAP but not in the labyrinth | 44 |
| Signaling molecules | |||||
| Wnt2 | E8.0-9.5 | Mes | 45 | Late gestation hemorrhage; tissue disruption in placenta | 45 |
| Wnt5a | E7.0, 8.0 | Mes | 46 | No affect on allantois | 46 |
| Wnt11 | E7.0-8.0 | Mes | 47 | Nd | |
| Rspo3 | E8.0, 8.5 | Mes | 48 | Vessels fail to invade placenta | 48,49 |
| Bmp4 | E8.25 | Mes | 50 | Small or no allantois | 51 |
| Smad1 | E7.25 | Mes | 52 | No chorio-allantoic fusion | 52 |
| Transcription factors | |||||
| Etv2 | E7.5, E8.25 | Mes | 53 | Lack of EC formation | 53 |
| Foxf1 | E8.25 | Mes | 54 | Absence of vasculature in allantois | 54 |
| FoxO1 | E8.0, E11.5 | EC of umbilical vessels | 55 | Lack of chorio-allantoic fusion | 56 |
| Snai1 | E8.25 | Mes | 31 | ECs fail to form plexus | 57 |
| dHAND | E8.25 | Mes | 31 | Nd | |
| T | E7.5-E8.5 | Mes | 58 | ECs fail to form plexus, lack of chorio-allantoic fusion | 2 |
| Tbx4 | E7.5-E10.5 | Mes (not EC) | 59 | ECs fail to form plexus, lack of chorio-allantoic fusion | 31,50 |
| Cdx2/Cdx4 | E7.5 | Mes | 60 | Decreased plexus formation, lack of chorio-allantoic fusion, vessels fail to invade placenta | 60 |
| Category/gene name . | Expression in the allantois or placenta . | Ref. . | Phenotype in allantois or placenta . | Ref. . | |
|---|---|---|---|---|---|
| Age . | Area of expression . | ||||
| Vegf signaling | |||||
| Vegf | E8.5, E12.5 | Mt, labyrinthine stroma | 19,20 | Degenerating endothelium in placenta | 21 |
| Flk1 | E7.5, E8.75, E12.5 | EC, placental vasculature | 5,19,20 | Lack of mature ECs in the allantois | 22 |
| Flt1 | E8.75, E12.5 | EC, placental vasculature | 5,19,20 | Nd | |
| EC | |||||
| Pecam | E8.0 onwards | EC | 5 | None (viable) | 23 |
| VE-Cad | E8.0 onwards | EC | 5 | No allantois plexus | 24-27 |
| Hematopoietic | |||||
| Tal1 | E7.0-8.0 | EPC | 5 | Absence of blood | 28 |
| Runx1 | E8.0-8.5 | Hematopoietic precursors | 7 | Nd | |
| SMC | |||||
| SM22α | E8.25 | SMC | None (viable) | 29 | |
| αSMA | E8.25 | SMC | Nd | ||
| ECM | |||||
| Fn | E8.25 | Mes* | 30 | Nd | |
| Itgα5 | E8.25 | Mes | 31 | Nd | |
| Collagen1α (I) | E8.25 | Mes | 31 | Nd | |
| Has2 | E8.25 | Mes | 31 | Nd | |
| Vcan | E8.25 | Mes | 31 | Nd | |
| Adamts9 | E7.5 | Mes | 32 | Nd | |
| Remodeling of vascular plexus | |||||
| Nrp1 | Nd | Nd | Nd | ||
| Nrp2 | Nd | Nd | Nd | ||
| Tie1 | E8.75, E12.5 | EC, placental vasculature | 19,20 | Nd | |
| Tie2 | E8.75, E12.5 | EC, placental vasculature | 19,20 | Nd | |
| Ang1 | Nd | Nd | Lack of complexity in umbilical vessels | 33 | |
| Ang2 | Nd | Nd | Nd | ||
| Tgfβ | E7.5-8.5 | Nd | 34 | Defective chorio-allantoic fusion; lack of Flk1 expression in allantois | 35 |
| Alk1 | Nd | Nd | Nd | ||
| Alk5 | Nd | EC and SMC of placenta | 36 | Defective sprouting of allantois into placenta; lack of SMCs around allantoic vessels | 36 |
| Edd | Nd | Nd | ECs fail to form plexus, lack of chorio-allantoic fusion | 37 | |
| Lpp3 | E8.0-E10.5 | Mes | 38 | ECs fail to form plexus, lack of chorio-allantoic fusion | 38 |
| Artery versus vein | |||||
| ephrinB2 | E9.5 | Vascular plexus, UA | 39 | None | 40 |
| EphrinB4 | E9.0 | EC/UV | 39 | Nd | |
| Notch2 | E8.25 | Mes | 31,41 | Nd | |
| Dll4 | E8.25 | Arterial EC | 31 | Degenerating vasculature in placenta | 42 |
| Jag1 | E8.25 | Mes | 31 | Nd | |
| Hey1 Hey2 | E8.5, E9.0 | Mes | 43 | Vasculature in CAP but not in the labyrinth | 44 |
| Signaling molecules | |||||
| Wnt2 | E8.0-9.5 | Mes | 45 | Late gestation hemorrhage; tissue disruption in placenta | 45 |
| Wnt5a | E7.0, 8.0 | Mes | 46 | No affect on allantois | 46 |
| Wnt11 | E7.0-8.0 | Mes | 47 | Nd | |
| Rspo3 | E8.0, 8.5 | Mes | 48 | Vessels fail to invade placenta | 48,49 |
| Bmp4 | E8.25 | Mes | 50 | Small or no allantois | 51 |
| Smad1 | E7.25 | Mes | 52 | No chorio-allantoic fusion | 52 |
| Transcription factors | |||||
| Etv2 | E7.5, E8.25 | Mes | 53 | Lack of EC formation | 53 |
| Foxf1 | E8.25 | Mes | 54 | Absence of vasculature in allantois | 54 |
| FoxO1 | E8.0, E11.5 | EC of umbilical vessels | 55 | Lack of chorio-allantoic fusion | 56 |
| Snai1 | E8.25 | Mes | 31 | ECs fail to form plexus | 57 |
| dHAND | E8.25 | Mes | 31 | Nd | |
| T | E7.5-E8.5 | Mes | 58 | ECs fail to form plexus, lack of chorio-allantoic fusion | 2 |
| Tbx4 | E7.5-E10.5 | Mes (not EC) | 59 | ECs fail to form plexus, lack of chorio-allantoic fusion | 31,50 |
| Cdx2/Cdx4 | E7.5 | Mes | 60 | Decreased plexus formation, lack of chorio-allantoic fusion, vessels fail to invade placenta | 60 |
CAP indicates chorio-allantoic plate; EC, endothelial cell; ECM, extracellular matrix; EPC, endothelial precursor cells; Mt, mesothelium; Mes, mesenchyme; Nd, not defined, SMC, smooth muscle cell; UA, umbilical artery; and UV, umbilical vein.
Mes refers to expression detected by whole mount and does not rule out possible expression in ECs.
Building the allantois vascular network: the known and the unknown
Vascular endothelial growth factor signaling and specification of ECs
The vascular endothelial growth factor (Vegf) signaling pathway lays the foundation for formation of ECs from mesoderm and also plays a major role in angiogenesis. Vegf and its tyrosine kinase receptor genes, Flk1 (also known as Kdr and Vegfr-2) and Flt1 (also known as Vegfr-1), are critical for the development of the vascular system. Flk1, which also marks other progenitors such as SMCs, is the earliest marker of EC lineage.6,61 In the early yolk sac, Flk1 marks the hemangioblasts, but later in development its expression is restricted to the EC lineage.62 In the allantois, Flk1 is expressed at the HF stage in angioblasts that will give rise to the vascular network.5 Both Flk1 and Flt1 are expressed in the allantoises of 9-16s stage embryos in putative angioblasts and nascent blood vessels.19 Vegf mRNA expression has been shown to be confined to the mesothelium of the allantois,20 whereas protein expression is localized to both mesothelial and core cells.19 Thus, similar to other sites in the embryonic vasculature, Vegf expression in the allantois is associated with active Flk1 expression and is suggestive of a role in maintenance of EC fate. Later in development, both Flk1 and Flt1 are expressed in the labyrinthine vasculature of the placenta and associated labyrinthine trophoblasts, whereas the labyrinthine stromal cells express Vegf.20
Loss of 1 allele of Vegf by targeted mutagenesis results in haploinsufficieny with embryonic lethality, affecting vasculogenesis and angiogenesis in both embryo and yolk sac.21,63 Although the development of the allantois vasculature was not studied in these mutants, it was suggested that the endothelial lining of the fetal placental vasculature degenerated.21 Targeted mutation of the Flk1 gene resulting in a null allele causes embryonic lethality at E9, with both embryo and allantois showing a lack of mature ECs.22 On the other hand, Flt1-null mutants show a disorganized embryonic and yolk sac vasculature and possibly an overgrowth of ECs, suggesting an inhibitory role for this receptor in vascular development.64 However, the allantois endothelium was not examined in Flt1 mutants. In addition, VEGF coreceptors neuropilin 1 (Nrp1) and neuropilin 2 (Nrp2) are important for vascular development. Embryos double mutant for Nrp1 and Nrp2 die around E8.5 and show an absence of vasculature in the yolk sac and embryo. However, the allantois was not examined in the double mutants, and the expression of Nrp1 and Nrp2 in the allantois has not been determined.65
The supporting mural cells of the allantois
Mural cells, which include pericytes and SMCs, support the developing vasculature and are characterized by the expression of platelet-derived growth factor receptor β (Pdgfrβ). The ligand for this receptor, PDGFB, is secreted by immature ECs and promotes the proliferation and expansion of mural cell progenitors.66 Both Pdgfrβ and Pdgfb-null mutants show defective smooth muscle recruitment in the embryo and the placenta, with disorganized and dilated vasculature.67 In addition to Pdgfrβ, vascular SMCs also express SM22α and αSMA.68 In the allantois, SM22α and αSMA expression begins at the 4-6s stage in the distal region and spreads proximally similar to the distal to proximal coalescence of ECs (R.A. and V.E.P., unpublished observations, 2012).
ECM in the allantois
ECM is essential during vessel formation for the adhesion, migration, proliferation, and vascular organization of ECs.69-73 Several ECM components are known to be important for development of the vasculature, but only a handful have been implicated in allantois vasculogenesis. fibronectin (Fn) and its binding partner α5 integrin (Itga5) are both necessary for EC tube formation. Both are expressed in the allantois, and mutations in either affect chorio-allantoic fusion,30,74 although the vasculature of mutant allantoises has not been analyzed. Embryonic stem cell-derived embryoid bodies (EBs) have been used as a model to study blood vessel formation. EBs lacking Fn or Itgα5 differentiate to form ECs but these fail to form tubular structures.74 Among the collagens, collagen 1 has been implicated in vascular development and is important for EC survival.75 Collagen1α (I) is expressed in the developing allantois,31 but its role in allantois vasculature has not been investigated.
In addition to matrix proteins, the proteoglycan HA, which is present in the allantois,76 is important for angiogenesis and, depending on the context, can act as a pro- or antiangiogenic agent.76-78 It is synthesized by hyaluronic acid synthase 2 (Has2), which is also expressed in the allantois,31,79 as is the chondroitin sulfate proteoglycan gene, versican (Vcan), a binding partner for HA.31 Mutation in either of these genes produces defects in endocardial cushion formation in the heart, but the allantois vasculature has not been analyzed.80,81
Another category of ECM molecules important for vascular morphogenesis is the proteinase family and its regulators, which include matrix metalloproteinases, TIMPs, ADAMs, and the ADAMTS family of proteins. In this category, only Adamts9 is known to be expressed in the allantois.32 ADAMTS9 is an EC-autonomous inhibitor of angiogenesis and also a tumor suppressor. Adamts9-null mutants die before gastrulation; hence, its role in vasculogenesis has not been analyzed.82
Signaling pathways involved in vascular remodeling
The angiopoietin-Tie signaling pathway has been implicated in branching, remodeling, and stabilization of the vascular plexus. The ligand genes angiopoietin1 (Ang1) and angiopoietin 2 (Ang2) and the endothelial-specific receptor tyrosine kinase genes, Tie1 and Tie2 (also known as Tek) interact to remodel the primary vascular plexus. Both Tie1 and Tie2 are expressed in the allantois at the 9-16s stage.19 Ang2 expression has been reported in the allantois at approximately the 30s stage83 but has not been investigated earlier; expression of Ang1 has not been investigated in the allantois. Both Tie284 and Ang133 homozygous-null mutants show dilation of blood vessels, defects in vascular remodeling, vessel sprouting, and branching, suggesting a role for these genes in EC interactions with the surrounding mesenchyme and ECM. Tie1-null mutants die at birth because of hemorrhage and lack of vascular integrity.84 Ang2 acts as a negative regulator of Ang1-Tie2 interactions. Ectopic expression of Ang2 under the control of the Tie2 promoter results in an angiogenic remodeling phenotype similar to the lack of Tie2 or Ang1.85 The allantois vasculature has not been analyzed in vivo in any of these mutants, although the umbilical vessels in the Ang1 nulls reportedly show a lack of complexity.33
The Tgfβ signaling pathway also has been implicated in vascular remodeling. Tgfβ and its downstream effectors activin receptor like kinase 1 (Alk1) and Alk5 (also known as TβR1) are all involved in angiogenesis.6 Tgfβ is expressed in the allantois,34 and null mutants for Tgfβ show defects in vascular network formation in the yolk sac and allantois. Some of the mutants also show an absence of chorio-allantoic fusion.35 ECs derived from Alk5-null mutant embryos show defective migration in in vitro assays. In addition, Alk5 mutants show defects in infiltration and sprouting of the allantois vasculature into the labyrinthine layer of the placenta.36
Signaling pathways involved in differentiation between arteries and veins
Differentiation of blood vessels into arteries or veins was once assumed to be the result of physical differences such as oxygenation, blood pressure, and shear forces, but there is some evidence from zebrafish that artery and vein distinction is inherently programmed.86 Two types of molecules, the ephrins and notch family molecules, have been identified as molecular markers for this differentiation process.6 EphrinB2 an Eph family ligand is expressed in the arterial system and conversely the receptor Eph-B4 is expressed in the venous system.39 Deficiency of either of these molecules leads to loss of angiogenesis in both arteries and veins, presumably because of a lack of interaction and thus lack of differentiation of the capillary beds.39,87 Similar to the embryonic and yolk sac vasculature, the ligand ephrinB2 is expressed in the umbilical artery and the receptor Eph-B4 is expressed in the umbilical vein.39
The notch pathway is activated downstream of VEGF signaling and induces arterial differentiation.88 In addition, it has been shown that notch family members Notch1, Notch3, Notch4, Delta-like 4 (Dll4), Jagged1 (Jag1), and Jagged2 are all expressed in arteries.89 Notch signaling lies upstream of ephrin signaling and has been implicated in driving arterial differentiation.90 In the allantois, Dll4 is restricted to the developing vessels,31 which will ultimately remodel to form the umbilical artery.91 Notch2 and Jag1 are expressed in the allantois mesenchyme.31,41 The death of Notch2-null mutants at E10.5 has been attributed to apoptosis in the embryo, although the allantois and placental vasculature were not analyzed41 and could play a contributing role. Loss of 1 allele of Dll4 results in lethal haploinsufficiency, with heterozygous mutants displaying degenerating vasculature in the placental labyrinth.42 Mutation in Jag1 leads to yolk sac and embryonic vasculature defects, but the allantois and placenta have not been analyzed in these mutants.92 The basic helix loop helix transcription factors Hey1 and Hey2 (also called Hes) are activated downstream of Notch signaling and act as transcriptional repressors. Both Hey1 and Hey2 are expressed in the allantois from E8.5.43 Loss of both Hey1 and Hey2 leads to a vascular phenotype, similar to the ephrinB2 ligand or Eph-B4 receptor–null mutants, where there is a defect in the remodeling of the vasculature. Indeed, Hey1 and Hey2 double mutants show a loss of ephrinB2 expression in vascular tissues. The allantois of double mutants attaches to the chorion and although the chorionic vessels form, they fail to invade the placental labyrinth.44
Dependence of vascular network formation on hedgehog signaling
Flk1-positive angioblasts of the visceral yolk sac organize into lumenized vascular structures in vitro only in the presence of a visceral endodermal layer, suggesting that signals from the endoderm are essential for vascular network formation.10 On the other hand, the yolk sac mesodermal layer differentiates into Flk1-positive angioblasts upon culture in the presence or absence of the adjacent endodermal layer.10 In the absence of endoderm, Ihh, one of the ligands for the hedgehog (Hh) signaling pathway, can induce a vasculogenic program in anterior epiblast cells in vitro.93 Furthermore, EBs lacking Ihh or smoothened (Smo), the receptor for Hh ligands, form Pecam-positive ECs that fail to form endothelial tubes.94 In addition, Hh signaling from the endoderm is essential to promote vascular network formation within the embryo,95 highlighting the role of Hh signaling in blood vessel formation in different vasculogenic systems. A Smo-null mutation, which results in abrogation of all Hh signaling, provides evidence that Hh signaling has different functions in development of the dorsal aorta, yolk sac, and allantoic vasculature: In the anterior region of Smo mutant embryos, Pecam-positive ECs fail to connect and cavitate to form the dorsal aorta96 ; in the yolk sac, ECs connect to form a primary vascular plexus but fail to remodel into a mature vascular network54 ; in the allantois, which lacks visceral endoderm, there is no apparent effect on vessel formation. Thus, the loss of Hh signaling affects vasculogenesis in the dorsal aorta, angiogenesis in the yolk sac, but neither of these processes in the allantois.
Hh signaling regulates vessel formation by regulation of the transcription factor Foxf1 and its downstream effector, Bmp4, in the yolk sac and developing embryo. However, Foxf1 expression is independent of Hh signaling in the posterior end of the primitive streak and the allantois, perhaps explaining why loss of Hh signaling does not affect allantois vasculogenesis. Allantois vascular development is nonetheless dependent on Foxf1 as mutations in Foxf1 produce vascular phenotypes in dorsal aorta, yolk sac and allantois.54
Wnt and Bmp signaling in blood vessel formation
The canonical Wnt family member, Wnt2, plays important roles in EC proliferation and vascular network formation in 2 in vitro systems, hepatic sinusoidal ECs97 and EBs.98 Wnt2 is expressed in the allantois throughout its growth and vascularization and Wnt2-null mutants show placental defects during late gestation.45 Endothelial-specific loss of β-catenin, the downstream effector of canonical Wnt signaling, affects vessel diameter in the yolk sac, umbilical vessels, and the cephalic plexus. In addition, the placentas from these mutants show a reduced number of fetal blood vessels in the labyrinthine layer.99 Rspo3, a secreted protein that can activate the canonical Wnt signaling pathway, is important for activation of Vegf.48 Rspo3 is expressed in the allantois, and its mutation leads to failure of the allantoic vessels to invade the placenta as well as vascular defects in the yolk sac.48,49
Noncanonical Wnt5a and Wnt11 affect EC proliferation, migration, and network formation in vitro.100-102 Both Wnt5a and Wnt11 expression have been observed in the allantois.31,46,47,103 Mutation of Wnt5a leads to a shortening of the A-P axis of the embryo, although it does not affect development of the allantois.46 Most Wnt11-null mutants are embryonic lethal before midgestation, but the cause of death has not been determined,103 and thus, Wnt11 might affect the development of allantois or placental vasculature.
Bmp4 is implicated in induction of mesoderm and differentiation of mesoderm into ECs.104 Loss of Bmp4 leads to a deficiency in the amount of mesoderm, resulting in aberrant yolk sac blood island formation and a small or absent allantois.51 Mutation of Smad1, a downstream effector of BMP signaling, results in defective chorio-allantoic fusion, but the vasculature of the allantois has not been analyzed.52
Transcription factors important for blood vessel formation
Several transcription factors are involved in EC and blood vessel formation in the embryo and yolk sac. A subset of these has been investigated in the allantois, including Etv2, Snai1, and dHAND. Etv2 (also known as ER71 or Etsrp71) is expressed in the allantois at E7.5 and E8.25. Etv2 is important for EC identity; Etv2 homozygous-null mutants are devoid of ECs in the embryo, yolk sac, amnion, and the allantois.53 In addition, in these mutants the placental labyrinthine layer is reduced in thickness and devoid of any vasculature. The zinc finger transcriptional repressor, Snai1, is important for mesoderm formation and its epiblast-specific deletion results in vascular defects throughout the embryo. ECs differentiate but fail to coalesce into a primary vascular plexus in the embryo or the allantois.57 dHAND, a basic helix loop helix transcription factor, is expressed in the developing vascular SMCs and their precursors. Lack of dHAND results in disorganized vasculature in the yolk sac and embryo, possibly because of regulation of Np1 by dHAND.105 dHAND is expressed in the allantois although the allantoises and the placentas in dHAND mutants have not been analyzed.106
T and Tbx4, 2 members of the T-box family of transcription factor genes, have been implicated in the process of vasculogenesis in the developing allantois. Both of these genes are expressed in the allantois starting E7.5. T is expressed until the 4-6s stage, whereas Tbx4 is expressed at least until the formation of umbilical vessels at E10.5.58,59 Loss of function mutation in either of these genes leads to absence of chorio-allantoic fusion and a lack of vascular plexus formation, although the ECs differentiate and express Pecam.2,31,50
Caudal related homeobox (Cdx) transcription factor genes Cdx2 and Cdx4 are both expressed in the allantois at E7.5.60 Heterozygous or homozygous loss of Cdx4 in a Cdx2 heterozygous background leads to defective development of the placental vasculature. A small percentage of Cdx4 null; Cdx2 heterozygous embryos show an absence of chorio-allantoic fusion; those allantoises that do fuse show a decreased organization of ECs or defective penetration of the allantois vasculature into the chorion leading to a nonfunctional placental labyrinth.60
Allantois culture ex vivo: advantages, limitations, and methods
The allantois has many advantages as a system to study blood vessel development. First, it can be isolated from the embryo and cultured ex vivo. Because the allantois contains mesodermal cells capable of differentiating into ECs in response to signals provided by the mesothelium, mesenchyme, and surrounding ECM, also present in the explant, it is a self-contained system with the potential to undergo vasculogenesis or angiogenesis in vitro.19,24 When isolated early (HF-1s stage), explants undergo vasculogenesis, and when isolated later (4-6s stage), undergo angiogenesis.107 Second, allantois explants can be imaged with the use of time-lapse microscopy to follow the sequence of events that occur during vessel formation in vitro.25 Finally, cultured allantois explants can be immunostained for markers of vessel formation and can be sectioned for histologic analysis.19,108 Culture methods for other areas of vasculogenesis in the embryo, including the lateral plate mesoderm and the yolk sac, have not been well established.
Hemodynamic blood flow plays an important role in remodeling, maturation, and arteriovenous specification of the embryonic and yolk sac vasculature.109,110 Such a role for hemodynamic forces has not been reported in the developing allantois vasculature and a limitation of allantois cultures is the absence of blood flow.
Adherent allantois culture
The most commonly used method to culture the allantois is in serum-containing media on tissue culture plastic or glass coated with poly-D-lysine or fibronectin for up to 72 hours.19,25,107 After 4-6 hours, the distal tip of the allantois attaches to the plastic or glass, and mesenchymal cells begin to spread. By 12 hours, the entire allantois is firmly attached. As occurs in vivo, the explant begins vascularization at the distal end of the allantois.19,107 The vascular plexus expands on top of and in communication with the mesenchymal layer of cells. By 18-20 hours of culture, the explant adopts a circular shape with the vascular plexus covering the entire area except at the periphery, which is populated only by mesenchymal cells (Figure 2).19,31,111 As shown histologically, vessels of the plexus form lumens in vitro.112 Explants show expression of all EC markers seen in vivo, namely Flk1, Flt-1, Tie1, Tie2, Pecam, and VE-Cad. The mesothelium of allantois explants can be identified with VCAM-1 staining and is present on top of the vascular plexus.19,108
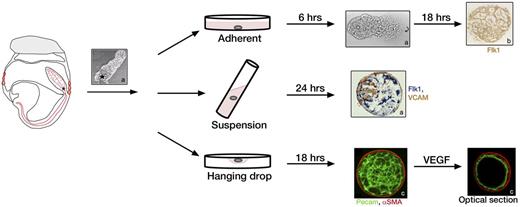
Three methods for allantois culture. Allantoises can be dissected from embryos from the HF to 4s stage and allowed to attach to glass, plastic, or filters for adherent cultures (top row), or they can be kept in suspension in rolling cultures (middle row) or hanging drops (bottom row). Asterisk indicates the proximal end of the allantois closest to the embryo. In adherent cultures the distal tip of the allantois attaches to the dish within 6 hours and a vascular plexus forms after 24 hours as indicated by staining with Flk1 (brown).31 Rolling cultures result in development of spheroids with 3 dimensional (3D) morphology, which can be stained whole as shown for Flk1 (blue) and VCAM (brown).19 Hanging drop cultures of allantoises also form 3D structures with a vascular plexus after 18 hours of culture. Image shows fluorescent staining of a whole mount allantois spheroid with α-SMA (red) and Pecam (green). After treatment with VEGF, the spheroid remodels into a single layer of Pecam-positive ECs surrounded by α-SMA–positive SMCs as shown in an optical section.111 Images a, b, and c are reprinted with permission from Downs et al,19 Arora et al,31 and Gentile et al,111 respectively.
Three methods for allantois culture. Allantoises can be dissected from embryos from the HF to 4s stage and allowed to attach to glass, plastic, or filters for adherent cultures (top row), or they can be kept in suspension in rolling cultures (middle row) or hanging drops (bottom row). Asterisk indicates the proximal end of the allantois closest to the embryo. In adherent cultures the distal tip of the allantois attaches to the dish within 6 hours and a vascular plexus forms after 24 hours as indicated by staining with Flk1 (brown).31 Rolling cultures result in development of spheroids with 3 dimensional (3D) morphology, which can be stained whole as shown for Flk1 (blue) and VCAM (brown).19 Hanging drop cultures of allantoises also form 3D structures with a vascular plexus after 18 hours of culture. Image shows fluorescent staining of a whole mount allantois spheroid with α-SMA (red) and Pecam (green). After treatment with VEGF, the spheroid remodels into a single layer of Pecam-positive ECs surrounded by α-SMA–positive SMCs as shown in an optical section.111 Images a, b, and c are reprinted with permission from Downs et al,19 Arora et al,31 and Gentile et al,111 respectively.
3D allantois culture
An alternative culture method that better preserves the 3-dimensional (3D) structure of the allantois is suspension culture in rolling tubes (Figure 2). With this method, isolated allantoises round up and develop a vascularized core surrounded by an outer layer of mesothelium. These 3D structures can be sectioned to study histomorphology and gene expression patterns in detail.19,108
Another method that preserves 3D structure is the hanging drop culture that generates allantoic spheroids (Figure 2). These spheroids contain a 3D network of Pecam-positive endothelial tubes and an outer layer that stains for SMC markers αSMA, SM22α and smooth muscle myosin. On addition of VEGF to the culture media, vessels in the core fuse to form uniluminal vascular spheroids characterized by an inner Pecam-positive EC layer and a closely associated outer layer of αSMA-positive cells. This culture system provides a means to study interactions between ECs and SMCs.111 Furthermore, when 2 uniluminal spheroids are placed in contact with one another in the presence of VEGF, the spheroids fuse to form a single large spheroid modeling the fusion of the paired dorsal aortae in the developing embryo.113
Allantois culture for the derivation of hematopoietic cells
Tal5 and CD41,8 markers for hematopoietic progenitors, and Runx1,7 a marker for the definitive hematopoietic lineage, are all expressed in the prefusion allantois. Despite the presence of these markers, it is not certain whether the allantois gives rise to hematopoietic cells in vivo. However, the allantois does have the potential to form hematopoietic cells in vitro. When multiple allantoises are cultured together for 2-5 days, dissociated to a single-cell suspension and seeded in media containing methylcellulose and cytokines, the cells show myeloid and erythroid differentiation in a colony-forming assay.7,8
Use of adherent allantois cultures to study gene function in blood vessel formation
VE-Cad function and signaling in blood vessel formation
VE-Cad is specifically expressed by ECs at cell-cell adherens junctions. Embryos that lack VE-Cad gene function show impaired vascular development; mutants die at E9.5 because of a failure of vessel remodeling and maturation.26 Although initial EC differentiation occurs, the mutant phenotype is complex, and which subsequent step of vasculogenesis is affected is the subject of differing opinions.26,27 One interpretation is that ECs in the allantois and yolk sac fail to form a vascular plexus; the alternative interpretation is that plexus formation proceeds normally but subsequent stabilization of the plexus fails, leading to its disintegration.24,27
Allantois cultures were used in 2 studies with different VE-Cad blocking antibodies (Abs) to address these alternative hypotheses. Culture of wild-type allantoises in the presence of a VE-Cad Abs results in a phenotype identical to culture of VE-Cad null mutant allantoises, that is, the presence of Pecam-positive ECs but absence of a vascular plexus.24 In the first study, early wild-type allantoises were cultured with VE-Cad Abs either during the early phase of culture followed by culture in the absence of VE-Cad Abs or vice versa. When the VE-Cad Ab was added during the initial phase of culture, vascular plexus formation was not affected. When the Ab was added in the later phase, the plexus showed disconnected clusters of ECs. These results were interpreted as a disassembly of the vascular plexus, which would suggest a role for VE-Cad in plexus stabilization, rather than plexus formation.24 However, an alternate explanation is that loss of VE-Cad function affects EC motility.
In a second study, this possibility was examined with the use of time-lapse imaging. Wild-type allantoises were cultured in the presence of Cy3-CD34, an antibody that immunolabels ECs without affecting vascular network formation, allowing tracking of individual ECs during the period of vessel formation.25 Using this method, EC movements can be studied in the presence of independent movement of the allantois mesenchyme and mesothelium, mimicking the in vivo scenario of blood vessel development. The major findings of this study were that new interconnections of ECs are formed (1) by gradual enlargement of an avascular area and (2) by vasculogenic sprouting involving initiation and extension of a vascular cord until it meets another cord. As a new sprout extends and interacts with the surrounding matrix, new ECs are added to the sprout from the EC cluster at the site of sprout initiation. Treatment with another VE-Cad Ab, different from the first study, does not prevent sprout initiation but prevents the addition of ECs to the extending sprout, without which the sprouts regress and fail to form new interconnections to make a plexus. In addition, movement of the ECs, independent of the movement of the mesenchymal or mesothelial layers, was reduced by 50% in the presence of VE-Cad Ab.25 Allantois culture in conjunction with live imaging has thus helped resolve the function of VE-Cad as being important for EC-autonomous motility and for contribution of ECs to sprouts during plexus formation. However, an additional role for VE-Cad in vascular plexus stability has not been ruled out, especially as distinct antibodies were used in the 2 studies.
VE-PTP function in blood vessel formation
Vascular endothelial protein tyrosine phosphatase (VE-PTP) is an EC–specific phosphatase that associates with membrane-bound Tie-2 and VE-Cad.114,115 Embryos lacking VE-PTP die at E10 because of lack of remodeling of the embryonic vasculature and aberrant enlargement of the yolk sac vessels.116 Addition of antibodies against VE-PTP in adherent allantois cultures leads to a similar enlargement of vessels and has thus been used to explore the mechanism of this abnormal enlargement of vessels.117 In allantois cultures, in the absence of Tie-2 receptor, VE-PTP antibodies fail to cause vessel enlargement; however, the addition of Ang1, which causes overactivation of Tie-2 signaling, leads to abnormally enlarged vessels. Furthermore, the absence of VE-PTP function and hence VE-PTP-bound Tie-2 causes abnormal activation of the Ang1-Tie2 signaling, leading to increased proliferation of ECs and resulting in enlarged vessels, suggesting that VE-PTP functions to regulate the levels of signaling through the Tie-2 receptor.117
Role of lipids in angiogenesis
Sphingosine-1-phosphate (S1P) is a phospholipid generated by the phosphorylation of sphingosine by sphingosine kinase. S1P binds to one of its receptors, S1P1-S1P5, and mediates signaling via heterotrimeric G-protein receptors.118 S1P plays a role in different processes important for blood vessel formation, including EC migration, chemotaxis, cytoskeletal reorganization, and adherence junction assembly,119 but, being a lipid, its precise role cannot be determined with the use of in vivo mutagenesis. S1P1 regulates vessel stabilization by recruitment of mural cells in vivo.120 S1P1, S1P3, and sphingosine kinase 2 are expressed in the allantois and embryo. Early allantoises (E7.8) cultured in serum-free conditions form ECs but fail to form a vascular plexus whereas allantoises cultured from later embryos (E8.5) show limited plexus forming ability. S1P rescues vascular plexus formation in these cultures by promoting expansion of the vascular network and the mesothelial layer without affecting cell survival. Thus, using the allantois as a model system revealed that S1P does not affect endothelial, angioblast or mesenchymal cell survival but instead affects the degree of expansion of the vascular network, suggesting a potential role in EC migration in vasculogenesis.119
Autotaxin (ATX) a protein involved in lipid metabolism converts lysophosphatidyl choline to lysophosphatidic acid (LPA) and sphingophosphocholine to S1P in vitro. Embryos homozygous for a mutation in the ATX gene Enpp2 show severe vascular defects whereas heterozygous adult mice show decreased circulating levels of LPA, suggesting ATX might function through the production of LPA in vivo. Vascular plexi of wild-type allantois explants readily disintegrate when serum is removed from the culture media. This disintegration can be prevented by addition of ATX or LPA. Thus, adherent allantois cultures provide evidence that these molecules are essential for maintenance of the vascular plexus.121
Other gene functions established with allantois culture
Deficiency of RA-GEF1, a guanine nucleotide exchange factor for the small GTPase Rap1, leads to impaired vascular development and decreased plexus formation in allantois cultures as measured by vessel sprout formation.122 ECs from RA-GEF1–null allantois explants show a decrease in VE-Cad expression and a lack of GTP bound RAP1. VE-Cad expression is rescued when the cells of the mutant allantois are transfected with a constitutively active, GTP-bound form of RAP1 and cultured with mouse stroma cells. In addition, when mutant allantoises are transfected with constitutively active RAP1 and cultured in collagen gels, an increase in vessel sprout formation is seen. Thus, the allantois culture system was used to show that RA-GEF1 regulates vascular network formation by controlling RAP1 activation and VE-Cad expression in ECs.122
Use of the adherent allantois culture system also has shown the function of PDGFB in vascular outgrowth,123 BMP signaling in SMC differentiation,54 histone deacetylase in commitment of progenitor cells to an EC fate,124 Gas1 in EC survival,125 and PI3K and ARAP3 in sprout formation126 (Table 2).24,25,54,116,117,119,121-129
Gene function identified using allantois culture as a model system
| Category/gene name . | Type of molecule . | LOF/GOF (method) . | Effect on allantois culture . | Inferred function . | Ref. . |
|---|---|---|---|---|---|
| VE-Cad function and signaling | |||||
| VE-Cad | Cell-cell adhesion | LOF (null mutant and Ab) | Disconnected ECs in plexus; loss of nuclear pSmad2/3 levels; expression of Survivin in all ECs | EC motility; addition of ECs to new sprouts; plexus stabilization; regulation of TGFβ signaling; restriction of Survivin expression to tip ECs | 24,25,127,128 |
| VE-PTP | Phosphatase | LOF (null mutant and Ab) | Excess EC coverage and simple monolayer architecture of ECs | Decreased EC proliferation | 116,117 |
| Gas1 | Cell cycle | LOF (null mutant) | Apoptosis of mesenchymal cells | EC survival | 125 |
| Lipid function | |||||
| Sphingosine-1-phosphate | Lipid | GOF (added to culture) | Promotes plexus formation in serum-deprived cultures | Promotes vasculogenesis and vascular outgrowth | 119 |
| Autotaxin | Lipid synthesis | GOF (added to culture) | Prevents plexus disintegration in serum-deprived conditions | Plexus stabilization | 121 |
| Lysophosphatidic acid | Lipid | GOF (added to culture) | Prevents plexus disintegration in serum deprived conditions | Plexus stabilization | 121 |
| PI3K signaling | |||||
| PI3K | Kinase | LOF (inhibitor) | Decrease in sprout formation | Angiogenesis | 126 |
| Arap3 | GTPase | LOF (null mutant) | Decrease in sprout formation | Angiogenesis | 126 |
| RA-GEF1 | GTPase | LOF (null mutant) | Failure to form vascular plexus | Controls activation of RAP1; controls induction of VE-Cad; promotes plexus formation | 122 |
| Pdgfb | Growth factor | GOF (GF in culture) | Promotes plexus formation in low serum conditions | EC survival or maintenance | 123 |
| Hdac | Deacetylase | LOF (inhibitor) | Decrease in vascular network formation | Increased commitment of progenitors to ECs | 124 |
| Bmp9 | Signaling | GOF (GF in culture) | Promotes formation of a disorganized plexus | Increased EC proliferation | 129 |
| Bmp4 | Signaling | LOF (inhibitor) | Decrease in plexus formation | Recruitment of mural cells that promote plexus stability | 54 |
| Category/gene name . | Type of molecule . | LOF/GOF (method) . | Effect on allantois culture . | Inferred function . | Ref. . |
|---|---|---|---|---|---|
| VE-Cad function and signaling | |||||
| VE-Cad | Cell-cell adhesion | LOF (null mutant and Ab) | Disconnected ECs in plexus; loss of nuclear pSmad2/3 levels; expression of Survivin in all ECs | EC motility; addition of ECs to new sprouts; plexus stabilization; regulation of TGFβ signaling; restriction of Survivin expression to tip ECs | 24,25,127,128 |
| VE-PTP | Phosphatase | LOF (null mutant and Ab) | Excess EC coverage and simple monolayer architecture of ECs | Decreased EC proliferation | 116,117 |
| Gas1 | Cell cycle | LOF (null mutant) | Apoptosis of mesenchymal cells | EC survival | 125 |
| Lipid function | |||||
| Sphingosine-1-phosphate | Lipid | GOF (added to culture) | Promotes plexus formation in serum-deprived cultures | Promotes vasculogenesis and vascular outgrowth | 119 |
| Autotaxin | Lipid synthesis | GOF (added to culture) | Prevents plexus disintegration in serum-deprived conditions | Plexus stabilization | 121 |
| Lysophosphatidic acid | Lipid | GOF (added to culture) | Prevents plexus disintegration in serum deprived conditions | Plexus stabilization | 121 |
| PI3K signaling | |||||
| PI3K | Kinase | LOF (inhibitor) | Decrease in sprout formation | Angiogenesis | 126 |
| Arap3 | GTPase | LOF (null mutant) | Decrease in sprout formation | Angiogenesis | 126 |
| RA-GEF1 | GTPase | LOF (null mutant) | Failure to form vascular plexus | Controls activation of RAP1; controls induction of VE-Cad; promotes plexus formation | 122 |
| Pdgfb | Growth factor | GOF (GF in culture) | Promotes plexus formation in low serum conditions | EC survival or maintenance | 123 |
| Hdac | Deacetylase | LOF (inhibitor) | Decrease in vascular network formation | Increased commitment of progenitors to ECs | 124 |
| Bmp9 | Signaling | GOF (GF in culture) | Promotes formation of a disorganized plexus | Increased EC proliferation | 129 |
| Bmp4 | Signaling | LOF (inhibitor) | Decrease in plexus formation | Recruitment of mural cells that promote plexus stability | 54 |
Ab indicates antibody; LOF, loss of function; GOF, gain of function; and GF, growth factor.
Applications in disease and therapeutics
Apart from its importance in normal embryonic development, blood vessel formation plays a key role in many pathologic conditions, such as cancer, diabetic retinopathy, rheumatoid arthritis, obesity, age-related macular degeneration, and neurologic disorders such as Parkinson and Alzheimer disease.130 Thus, systems that model blood vessel formation are valuable not only for understanding basic mechanisms of vessel formation but also for screening drugs that influence vessel formation. Although in vitro model systems are available that use cultured EC lines, these models have limitations. Being homogenous EC cultures, they lack the presence of any mural cells or ECM that provides a tissue-type environment.
Allantois cultures, on the other hand, contain all the cellular and extracellular components that support vessel formation. Thus, the effect of drugs or small molecules on all the processes of vessel formation—EC proliferation, EC migration, endothelial tube formation, and formation of an EC vascular network—can be determined in a context that closely mimics in vivo vessel formation. For example, the PI3K inhibitor PI-103, which inhibits vascularization of human tumor xenografts,131 also inhibits vascular network formation in the adherent allantois culture.126 In addition, the allantois culture system is robust and reproducible. Methods for quantification of different parameters in this system are now available.132 Because there is no standard model that can mimic an in vivo disease situation completely, the combined use of EC cell lines and allantois explants offers a good drug-screening strategy to study the effects of the drugs on blood vessel formation.
Conclusions
The use of the allantois to study blood vessel formation is in its infancy, although the studies reviewed here amply illustrate its usefulness. The allantois culture methodology allows teasing out function when a vascular defect results in early lethality and it can be used to distinguish if a vascular phenotype is primary or secondary to the loss of gene function elsewhere in the embryo. Finally, allantois explant culture provides an additional in vitro system for drug screening and establishing drug efficacy as pro- or antiangiogenic. Thus, the allantois is emerging as an important tool to study vasculogenesis and angiogenesis in development and disease.
Acknowledgments
The authors thank Jan Kitajewski for the critical reading of the manuscript.
This work was supported by grant R37HD033082 (to V.E.P.) from the Eunice Kennedy Shriver NICHD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD.
National Institutes of Health
Authorship
Contribution: R.A. and V.E.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginia Papaioannou, Department of Genetics and Development, Columbia University Medical Center, 701 W 168th St, New York, NY 10032; e-mail vep1@columbia.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal