Abstract
Histiocytoses are rare disorders of unknown origin with highly heterogeneous prognosis. BRAF mutations have been observed in Langerhans cell histiocytosis (LCH). We investigated the frequency of BRAF mutations in several types of histiocytoses. Histology from 127 patients with histiocytoses were reviewed. Detection of BRAFV600 mutations was performed by pyrosequencing of DNA extracted from paraffin embedded samples. Diagnoses of Erdheim-Chester disease (ECD), LCH, Rosai-Dorfman disease, juvenile xanthogranuloma, histiocytic sarcoma, xanthoma disseminatum, interdigitating dendritic cell sarcoma, and necrobiotic xanthogranuloma were performed in 46, 39, 23, 12, 3, 2, 1, and 1 patients, respectively. BRAF status was obtained in 93 cases. BRAFV600E mutations were detected in 13 of 24 (54%) ECD, 11 of 29 (38%) LCH, and none of the other histiocytoses. Four patients with ECD died of disease. The high frequency of BRAFV600E in LCH and ECD suggests a common origin of these diseases. Treatment with vemurafenib should be investigated in patients with malignant BRAFV600E histiocytosis.
Introduction
Histiocytoses encompass a wide range of rare and heterogeneous diseases characterized by the accumulation and/or the proliferation of histiocytes within various tissues. Since 1987, the classification for histiocytoses relies on the Langerhans and non-Langerhans cell origin.1 The distinction was based on the presence of Birbeck granules and, more recently, on CD1a expression on formalin-fixed, paraffin-embedded samples.2,3 The latest World Health Organization classifications has individualized Langerhans cell histiocytosis (LCH), Rosai-Dorfman disease, disseminated juvenile xanthogranuloma (JXG; synonym of Erdheim-Chester disease [ECD] and xanthoma disseminatum), interdigitating dendritic cell sarcoma, and histiocytic sarcomas.4,5 Diagnosis of these conditions is mainly based on histopathology and corresponds to highly variable clinical syndromes, whose prognoses range from benign self-healing to highly malignant.
The RAS-RAF-MEK-ERK pathway is a cellular signaling pathway, which plays a major role in tumors.6 BRAFV600E mutation, an activating mutation of the proto-oncogene BRAF, is present in several human tumors.7 This mutation results in an activation of RAS-ERK pathway, independently of RAS activation. Inhibition of BRAF activation by vemurafenib improves survival of patients with BRAFV600E metastatic melanomas.8 BRAFV600E mutations have been detected in patients with LCH.9,10 We thus investigated whether this mutation was present in other subsets of histiocytoses.
Methods
Patients and samples
Patients were retrieved from the databases of the French Registry of Histiocytoses, and of 3 teaching hospitals (Pitié-Salpêtrière, Necker-Enfants Malades, and Ambroise Paré). Thirty-nine and 12 consecutive cases of LCH and cutaneous JXG were included, respectively. For other histicytoses, all cases available were included. Study was approved by the ethic committee Ile de France III (#2011-A00447–34) and conducted in accordance with the Declaration of Helsinki. Clinical follow-up of patients with LCH and ECD was prospectively recorded according to previously described methodologies.11,12
All tissue samples were reviewed by at least 4 independent pathologists, trained in the field of histiocytoses (N.B., D.C., F.C., and J.-F.E.), and classified according to the World Health Organization classification.4,5 Immunohistochemistry was performed with CD1a (Beckman-Coulter), CD68 (Dako Denmark), CD163 (Thermo Scientific), CD205 (Langerin; Novocastra), S100 protein (Dako Denmark), and factor XIIIa (Novocastra) when necessary. For ECD cases and a few other difficult cases, the diagnoses were achieved taking into account the clinical and radiologic aspects of the disease.10 Patients with both LCH and ECD features were excluded.
Detection of BRAFV600 mutations
Tumor DNA was extracted from formalin-fixed, paraffin-embedded tissues as described.13 Four serial sections were performed for each sample. The first section was used for histology and selection of the areas of highest histiocyte density and the 3 others for dissection at ×10 magnification. When histiocyte infiltration was lower than 20%, sensitivity was considered as insufficient for BRAF heterozygous mutation detection. Detection of BRAF V600 mutations was performed by pyrosequencing with PyroMark Q24 (QIAGEN).
Immunochemistry with BRAFV600E
Mouse monoclonal antibody VE1 was shown to be specific of BRAFV600E mutation.14 Stainings were performed with Bond-Max (Leica Biosystems). Antigen retrieval was performed during 60 mn at 96°C in pH9 buffer Bond Epitope Retrieval Solution 2 (Leica Biosystems). VE1 hybridoma supernatant was diluted one-third and incubated at 37°C for 32 mn. Staining was revealed with Bond polymer refine red detection kit (Leica Biosystems). Staining was scored according to previously published criteria14 by a pathologist who was not aware of genetic results.
Statistical analysis
Differences between groups of patients were tested using Mann-Whitney or Kruskal-Wallis tests for continuous data, and Fisher exact or χ2 tests for categorical data. These analyses were followed by Bonferroni correction for multiple testing, when needed. All P values were 2-tailed, and statistical significance was defined as P < .05. Statistical analyses were performed using JMP8 (SAS Institute).
Results and discussion
Samples of the 127 patients mainly originated from bone (n = 29), skin (n = 27), lymph node (n = 18), perirenal infiltration (n = 12), and lung (n = 9; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Diagnosis of ECD (n = 46), LCH (n = 39), Rosai-Dorfman disease (n = 23), JXG (n = 12), histiocytic sarcomas (n = 3), xanthoma disseminatum (n = 2), interdigitating dendritic cell sarcoma (n = 1), and NXG (n = 1) were established. In all ECD cases, histiocytes were CD68+, CD1a−, S100−. Histiocytes were positive for factor XIII in 5 of 9 of ECD cases. All LCH cases contained CD1a+, S100+ histiocytes. BRAF mutational status was obtained by pyrosequencing in 93 (73%) of available cases. Failure to determine BRAF status was more frequent in bone and perirenal fat than in other sites of biopsies (49% vs 17%, P = .0005; supplemental Tables 1-3).
A BRAFV600E mutation was detected by pyrosequencing in 13 of 24 (54%) patients with ECD. For 2 patients, 2 different samples were available and both harbored the BRAFV600E mutation. The exact frequency of BRAF mutations in ECD patients remains to be confirmed in other series, as the present series may be biased by the fact that only 52% could be evaluated for BRAF status. The pathophysiology of histiocytoses remains to be determined. LCH was shown to be a clonal proliferation by HUMARA15 ; this result was confirmed with other methods. By contrast, there is an ongoing debate as to whether ECD should be considered as a tumor or an abnormal immune response. Indeed, the complex network of cytokines and chemokines associated with ECD underlines an intense systemic Th-1–oriented immune activation.16 However, ECD was shown to be clonal in 5 patients, with either HUMARA17 or cytogenetic.18 The detection in the present study of BRAF mutations in 13 other ECD cases confirms that ECD is a clonal proliferation.
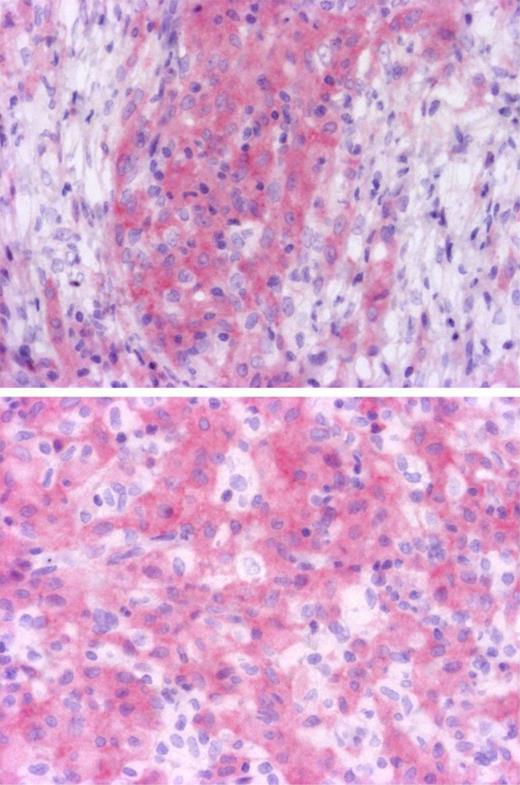
To determine which cells were mutated in ECD and further confirm the presence of the BRAFV600E mutation, we performed confirmatory immunohistochemistry analysis with BRAFV600E specific antibody on wild-type and mutated ECD samples. We recently confirmed in a series of melanomas that VE1 antibody was highly specific of BRAFV600E mutation, and shown it was more sensitive than Sanger sequencing (E. Colomba, Z.H.-R., A.v.D., C. Marin, N. Terrones, D. Pechaud, S. Surel, J.-F. Côté, F. Peschaud, D. Capper, H. Blons, U. Zimmermann, T. Clerici, P. Saiag, J.-F.E., Detection of BRAF p.V600E mutations in melanomas: comparison of four methods argues for sequential use of immunohistochemistry and pyrosequencing, manuscript submitted, August 10, 2012). Seven positive and 6 negative ECD cases identified with pyrosequencing were tested, and immunohistochemistry with VE1 confirmed the BRAF status in all cases. Only histiocytes were stained, whereas lymphocytes, fibroblasts, and endothelial cells were negative (Figure 1). Both mononucleated histiocytes and Touton cells were positive, confirming that both mononucleated and multinucleated histiocytes derive from the same tumor progenitor. In some areas, BRAF-negative histiocytes were admixed with positive cells (Figure 1), probably corresponding to reactive inflammatory cells. Validation of the detection of BRAF mutations with immunohistochemistry for diagnostic use would be helpful for cases with low histiocyte infiltration.
Identification of cells with BRAF mutation. Immunohistochemistry with BRAFV600E specific antibody VE1 disclosed cytoplasmic staining of histiocytes, whereas lymphocytes and fibroblasts were negative (top, original magnification ×100). Some histiocytes were not stained and correspond to reactive macrophages (bottom, original magnification ×200). Microphotographs were performed with a microscope BX41, eyepiece (WH 10×/22), objectives Olympus UPlanFI 10× and Olympus UPlanFI 20× (Olympus), Camera Axopcam ICc1, and AxioVision Rel Version 4.8 software (Carl Zeiss).
Identification of cells with BRAF mutation. Immunohistochemistry with BRAFV600E specific antibody VE1 disclosed cytoplasmic staining of histiocytes, whereas lymphocytes and fibroblasts were negative (top, original magnification ×100). Some histiocytes were not stained and correspond to reactive macrophages (bottom, original magnification ×200). Microphotographs were performed with a microscope BX41, eyepiece (WH 10×/22), objectives Olympus UPlanFI 10× and Olympus UPlanFI 20× (Olympus), Camera Axopcam ICc1, and AxioVision Rel Version 4.8 software (Carl Zeiss).
A BRAFV600E mutation was detected in 11 of 29 (38%) patients with LCH. This frequency was not statistically different from 13 of 24 (54%) that we observed in patients with ECD nor from 35 of 61 (57%) in the Badalian-Very series.9 No mutations were detected in patients with Rosai-Dorfman disease (n = 23), cutaneous JXG (n = 12), histiocytic sarcomas (n = 3), xanthoma disseminatum (n = 2), interdigitating dendritic cell sarcoma (n = 1), or NXG (n = 1). Thus, ECD and LCH share similar oncogenic pathways, which are distinct from other histiocytoses. Interestingly, associations of ECD and LCH have been reported,19 suggesting that both proliferations could derive from a common progenitor.
The treatment of ECD and LCH remains a challenge. Although some forms of LCH are benign and self-healing, some patients with ECD and LCH are resistant to several lines of chemotherapies.12,20 Disease-related death occurred in 6 of 46 ECD and 3 of 39 LCH cases. Within this small series, the clinical characteristics of ECD patients did not appear to depend on BRAF status (Table 1); however, this outcome should be checked in a larger series.
Main clinical characteristics of the 46 patients with ECD according to BRAFV600E status
| . | WT (n = 11) . | BRAF V600E (n = 13) . | NA (n = 22) . | P . | |
|---|---|---|---|---|---|
| BRAF V600E versus WT* . | Across all 3 categories† . | ||||
| Median age at diagnosis, y (range) | 55 (39-81) | 55 (37-72) | 57 (16-73) | .62 | .83 |
| Sex, male/female | 9/2 | 8/5 | 16/6 | .28 | .54 |
| Involvement | |||||
| CNS, n (%) | 2 (18) | 6 (46) | 11 (50) | .15 | .20 |
| Heart, n (%) | 4 (36) | 7 (54) | 10 (45) | .39 | .73 |
| Large vessels, n (%) | 5 (45) | 11 (85) | 14 (64) | .04 | .13 |
| Exophthalmos, n (%) | 3 (27) | 7 (54) | 7 (32) | .19 | .32 |
| Diabetes insipidus, n (%) | 2 (18) | 3 (23) | 7 (32) | .77 | .67 |
| Lung, n (%) | 4 (36) | 4 (31) | 10 (45) | .77 | .67 |
| Perirenal infiltration, n (%) | 3 (27) | 7 (54) | 13 (59) | .19 | .21 |
| Xanthelasma, n (%) | 4 (36) | 4 (31) | 6 (27) | .77 | .87 |
| Bone pain, n (%) | 5 (45) | 7 (54) | 11 (50) | .68 | .92 |
| Death of disease progression, n (%) | 2 (18) | 2 (15) | 5 (23) | .85 | .86 |
| . | WT (n = 11) . | BRAF V600E (n = 13) . | NA (n = 22) . | P . | |
|---|---|---|---|---|---|
| BRAF V600E versus WT* . | Across all 3 categories† . | ||||
| Median age at diagnosis, y (range) | 55 (39-81) | 55 (37-72) | 57 (16-73) | .62 | .83 |
| Sex, male/female | 9/2 | 8/5 | 16/6 | .28 | .54 |
| Involvement | |||||
| CNS, n (%) | 2 (18) | 6 (46) | 11 (50) | .15 | .20 |
| Heart, n (%) | 4 (36) | 7 (54) | 10 (45) | .39 | .73 |
| Large vessels, n (%) | 5 (45) | 11 (85) | 14 (64) | .04 | .13 |
| Exophthalmos, n (%) | 3 (27) | 7 (54) | 7 (32) | .19 | .32 |
| Diabetes insipidus, n (%) | 2 (18) | 3 (23) | 7 (32) | .77 | .67 |
| Lung, n (%) | 4 (36) | 4 (31) | 10 (45) | .77 | .67 |
| Perirenal infiltration, n (%) | 3 (27) | 7 (54) | 13 (59) | .19 | .21 |
| Xanthelasma, n (%) | 4 (36) | 4 (31) | 6 (27) | .77 | .87 |
| Bone pain, n (%) | 5 (45) | 7 (54) | 11 (50) | .68 | .92 |
| Death of disease progression, n (%) | 2 (18) | 2 (15) | 5 (23) | .85 | .86 |
P values computed using Mann-Whitney test, χ2 test, or Fisher test, as appropriate.
P values computed using Kruskal-Wallis test or χ2 test, as appropriate. None of these P values remains significant after Bonferroni correction for multiple testing.
Targeted therapies have recently been tested in both conditions21,22 ; however, it has limited efficacy. Prognosis of ECD has been substantially improved by IFN-α therapy, but many refractory forms subsist, especially those with CNS and cardiovascular involvements. Verumafenib, an inhibitor of BRAF, was recently approved for treating patients with metastatic melanoma and BRAFV600 mutations.8 The poor prognosis of a substantial number of patients with multisystemic ECD and LCH warrants new therapeutic approaches that could involve BRAF inhibitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dominique Peschaud, Gladwys Faucher, Nathalie Terrones, Mariama Bakari, Yolaine Pothin, Sylvie Surel, and Catherine Le Gall for contributing to the BRAF mutation analyses.
This work was supported in part by Ligue Contre le Cancer, Association pour la Recherche et l'Enseignement en Pathologie (AREP), and Association pour la Recherche en Oncologie Digestive (AROLD) nonprofit associations.
Authorship
Contribution: J.H., F.C., J.D., Z.A., and J.-F.E. designed research; J.H., F.C., L.A., Z.H.-R., B.H., F.C.-A., D.L., A.L., K.M., D.C., L.G., C.R., M.S., S.C., M.K., M.-C.C., S.F., N.B., Z.A., J.D., and J.-F.E. collected data; L.A. performed statistical analysis; J.H., F.C., Z.A., and J.-F.E. analyzed and interpreted data; A.v.D., F.S., and J.-F.E. performed anti-VE1 immunohistochemical analysis; J.H., F.C., Z.H.-R., and J.-F.E. analyzed data; J.H., F.C., L.A., Z.A., and J.-F.E. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: J.-F.E. received honoraria for counseling on diagnosis and/or treatment with BRAF inhibitors of patients with melanomas from Roche and Glaxo Smith Kline. The laboratory of J.-F.E. received grants from Roche for organization and external quality control assessment of BRAF mutation detection in France. D.C. and A.v.D. applied for a patent on the diagnostic use of BRAF V600E mutant-specific antibody VE1. All terms are being managed by the German Cancer Research Center in accordance with its conflict of interest policies. The remaining authors declare no competing financial interests.
Correspondence: Jean-François Emile, Pathology Department, Ambroise Paré Hospital, 9 Av. Charles de Gaulle, F-92104 Boulogne, France; e-mail: jean-francois.emile@apr.aphp.fr.
References
Author notes
F.C. and L.A. contributed equally to this study.