In this issue of Blood, Almeida et al report immediate benefits of hydroxyurea (HU) acute administration in diminishing vaso-occlusive processes in sickle cell disease (SCD) mice.1
Sickle cell disease is a recessive hereditary disorder because of a single amino acid substitution (Glu6Val) in the β globin gene. Mutated hemoglobin (HbS) polymerizes under hypoxic conditions leading to HbS fiber formation and red blood cell (RBC) sickling. SCD is characterized by painful episodic vaso-occlusive crises (VOCs), acute chest syndrome, and a chronic inflammatory state.
The process leading to vaso-occlusion in SCD is complex and poorly understood. While VOCs were commonly considered as a direct consequence of RBC sickling and adhesion, it is now clear that they involve multiple actors including leukocytes, platelets, endothelial cells, elevated levels of proinflammatory cytokines, oxidative stress, and reduced nitric oxide (NO) availability.2
HU, also called hydroxycarbamide, is currently the only drug having demonstrated benefit for SCD patients, with fewer VOCs and lower mortality and morbidity.3 HU is thought to act by increasing fetal hemoglobin (HbF) production in erythrocytes, leading to less HbS polymerization. However, several reports showed that HU could also act through other mechanisms, such as modulation of adhesion molecule expression, reductions in leukocyte counts and increased erythrocyte cation transport. Recently, Bartolucci et al showed that HU could reduce human sickle RBC adhesion to laminin through a decrease of intracellular cAMP, only 15 days after the treatment started.4 They provided evidence that HU could modulate the activation state of erythroid adhesion molecules independently of their expression level.4 HU is also suggested to act as a donor of NO in vitro, and to induce γ-globin expression via the NO second messenger cGMP.5 Consistent with these findings, Canalli et al showed that neutrophils from HU-treated patients had higher cGMP levels than untreated patients, with a lower adhesion to fibronectin and ICAM-1.6
Efforts have focused on VOCs in SCD. In 2002, Turhan and colleagues uncovered a new paradigm for the pathogenesis of sickle cell vaso-occlusion where adherent leukocytes play a direct role.7 They established an inflammatory model of VOC by treating mice expressing sickle hemoglobin with TNF-α, a cytokine that induces P- and E-selectin–mediated leukocyte rolling. Using intravital microscopy, they demonstrated that sickle RBCs bound to adherent leukocytes, producing vaso-occlusion of inflamed cremasteric venules.7 Work from the same group later identified neutrophils as the primary white blood cells (WBCs) involved in this process and showed that VOCs could be reversed by intravenous immunoglobulins or a synthetic pan-selectin inhibitor, GMI-1070, targeting neutrophil adhesion to activated endothelium.8,9 The importance of selectins in this process has been recently highlighted by Gutsaeva et al who showed that an anti–P-selectin aptamer efficiently inhibited RBC and leukocyte adhesion to endothelial cells in SCD mice.10
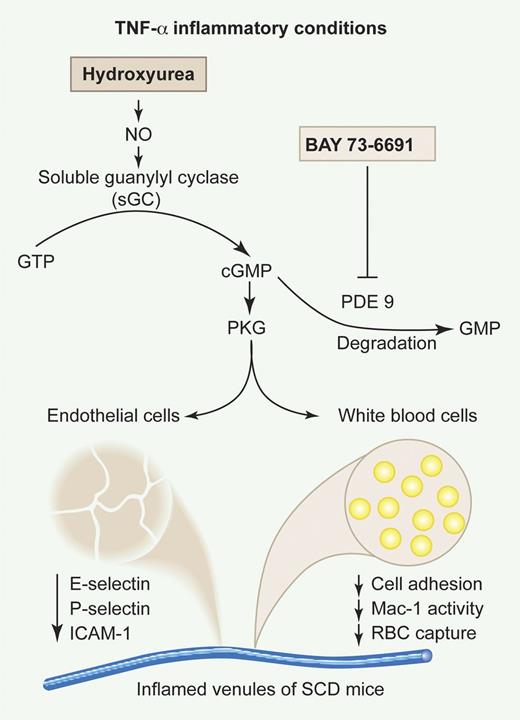
Almeida et al investigated the in vivo modulation of cGMP-dependent signaling by HU as a novel therapeutic approach to reduce leukocyte-endothelial interactions.1 They found that HU provoked significant reductions in leukocyte adhesion and extravasation, only 3 hours after its concomitant administration with TNF-α in SCD mice. Similar results were obtained with BAY73-6691, a specific inhibitor of the cGMP-degrading enzyme phosphodiesterase 9 (PDE9). Coadministration of both HU and BAY73-6691 was associated with an increase of plasma cGMP levels and led to a more pronounced anti-inflammatory effect than with either drug alone. The authors investigated the effects of HU and BAY73-6691 on endothelial and leukocyte activation and showed that they abrogated the TNF-α–induced up-regulation of 3 major endothelial adhesion molecules (ICAM-1, P-selectin, and E-selectin) in the cremaster muscle. HU and BAY73-6691 also led to a decrease in the activity of the leukocyte integrin Mac-1 (αMβ2) and in the heterotypic WBC-RBC interactions. Finally, the results obtained with a set of inhibitors strongly suggest that HU acts as a NO donor in this inflammatory scenario, activating a cGMP-dependent pathway that is synergistically amplified by BAY73-6691 (see figure).
Effects of HU and the PDE9 inhibitor BAY73-6691 on TNF-α–induced vaso-occlusion in SCD mice. Adapted from Figure 1 of Almeida et al, which begins on page 2879. Professional illustration by Paulette Dennis.
Effects of HU and the PDE9 inhibitor BAY73-6691 on TNF-α–induced vaso-occlusion in SCD mice. Adapted from Figure 1 of Almeida et al, which begins on page 2879. Professional illustration by Paulette Dennis.
The findings of the Almeida et al study are of a particular interest as they reveal for the first time a rapid anti-occlusive effect of HU. Interestingly, this effect was obtained with either intravenous or oral administration of the drug, which is promising for a potential oral use of HU by SCD patients to attenuate and potentially reverse vaso-occlusive episodes. The results obtained with the inhibitor BAY73-6691 point out the PDE9 enzyme as an attractive therapeutic target, because of its relatively restricted tissue distribution. However, the beneficial inhibitory effect of HU and BAY73-6691, demonstrated when these agents were administered at the time of TNF-α inflammatory induction, was not obtained once the vaso-occlusive events were already inaugurated. Future studies are needed to determine the drug doses required for the reversal of vaso-occlusion, once VOCs are established. Although approximately 25% of SCD patients are poor or nonresponders to a HU long-term treatment, this study opens new perspectives for the use of HU as an acute treatment of VOCs in SCD.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal