Abstract
We determined whether polymorphisms in Fcγ receptor (FcγR) IIa or FcγRIIIa genes were associated with outcomes in Vax004, a trial testing recombinant gp120 vaccination in preventing sexually acquired HIV infection. Male subjects (n = 1725), including infected and uninfected vaccinees and placebo recipients, were genotyped. We observed no association between FcγRIIa genotype and infection rate in vaccinees or placebo recipients. However, FcγRIIIa genotype was associated with infection rate among vaccinees (P = .035). Exploratory analyses revealed that vaccinees homozygous for the FcγRIIIa V allele in the lowest behavioral risk group had a greater rate of infection than low risk vaccinees with at least 1 F allele (hazard ratio [HR] = 3.52; P = .002). No such association was seen among vaccinees with high-risk behaviors or among placebo recipients in either risk stratum. Vaccinated low-risk VV subjects had a greater infection rate than low-risk VV placebo recipients (HR = 4.51; P = .17) or low-risk placebo recipients with any genotype (HR = 4.72; P = .002). Moreover, low-risk VV vaccinees had infection rates similar to individuals with high behavioral risk, irrespective of genotype. Our results generate the hypothesis that recombinant gp120 vaccine may have increased the likelihood of acquiring HIV infection in individuals with the VV genotype (present in ∼ 10% of the population) at low behavioral risk of infection.
Introduction
The RV144 vaccine trial in Thailand, in which investigators used bivalent recombinant gp120 (rgp120) in alum after a canarypox (ALVAC)–based prime, revealed a very modest level of protection from primarily heterosexually acquired HIV infection.1 Two trials that preceded RV144—Vax003, conducted in Thailand among intravenous drug users, and Vax004, conducted mainly in North America among men who have sex with men—demonstrated that rgp120 vaccines without ALVAC priming were unable to prevent HIV infection.2,3 However, secondary analyses of Vax004 suggested that vaccine-induced antibody responses might have influenced the risk of infection among study participants.4 In those analyses, infection rates decreased as levels of HIV-1–specific antibodies, measured in several assays, increased. In addition, vaccinees in the lowest quartile of antibody responses had a greater risk of infection than recipients of placebo.
Much of the biologic activity of antibodies results from interactions between the Fc segment of antibody and Fc receptors on cells such as monocytes, macrophages, dendritic cells, and natural killer (NK) cells. Several varieties of Fc receptors for IgG (FcγRs) have been described that differ with respect to their binding to Fc, their expression on different cell types, their mechanism of signal transduction, and the biologic functions that they mediate.5,6 Two of the receptors, FcγRIIa and FcγRIIIa, are each encoded by 2 alleles that impart phenotypic differences.6-9 A single nucleotide polymorphism in the gene for FcγRIIa results in either a histidine (H) or arginine (R) at amino acid position 131.7,8 The H isoform of the receptor has greater binding avidity to IgG2 and IgG3 immune complexes than the R isoform.6 FcγRIIa genotypes are associated with susceptibility to or severity of certain autoimmune and infectious diseases and with outcomes of monoclonal antibody cancer treatments.10-14 FcγRIIIa is encoded by alleles that confer either a phenylalanine (F) or valine (V) at amino acid position 158.9 The V isoform is reported to have greater affinity to monomeric IgG1 and IgG3 than the F receptor and also binds better to IgG1, IgG2, and IgG3 immune complexes.6 Like FcγRIIa, the FcγRIIIa polymorphism has been associated with susceptibility to or severity of autoimmune and infectious disease and with outcomes of antitumor monoclonal antibody therapy.10,13,15-17
In Vax004, antibody-dependent, cell-mediated virus inhibition (ADCVI) activity in serum correlated inversely with the rate of HIV infection.18 ADCVI is a measure of virus inhibition mediated by antibody and FcγR-bearing effector cells. Although FcγR-mediated immune functions, such as those measured in the ADCVI assay, are likely to be of benefit in preventing HIV infection, antibodies also may enhance infection through FcγRs. Antibody-dependent enhancement (ADE) has been demonstrated in vitro for HIV-1 and for many other viruses.19-22 In humans, the best evidence of ADE in vivo comes from work with dengue virus.23
The link between antibody responses and the risk of HIV infection in the Vax004 trial, the importance of Fc-FcγR interactions to antibody function, and the phenotypic differences in the genotypes of FcγRIIa and FcγRIIIa, led us to explore the relationship between FcγR polymorphisms and outcomes in Vax004.
Methods
Research involving human subjects was approved by the University of California, Irvine Institutional Review Board. All human specimens were processed without personal identifiers, and informed consent was waived by the Institutional Review Board. This study was conducted in accordance with the Declaration of Helsinki.
Subjects
We evaluated FcγR genotypes and their relationship to risk of infection in vaccinees and recipients of placebo who participated in the Vax004 trial. The Vax004 trial was a randomized, double-blind, placebo-controlled phase 3 trial designed to test the efficacy of an rgp120 vaccine in preventing sexually acquired HIV infection.3 Subjects in Vax004 were 5403 healthy volunteers (5095 men who have sex with men and 308 women at high risk of heterosexual transmission) who were randomized to satisfy a 2:1 vaccinee-to-placebo recipient ratio.1 A total of 1759 subjects, including 223 vaccinees who became infected during the trial, 1276 vaccinees who remained uninfected, 108 infected placebo recipients, and 152 uninfected placebo recipients, were genotyped at one or both FcγR gene loci. The sample included all infected individuals from whom adequate specimen for genotyping was available. For uninfected vaccinees, 2 random samples were chosen; the first consisted of 163 subjects and the second, to gain power, consisted of 1088 subjects. The second group included only male subjects. A third group of uninfected subjects (126 vaccinees and 40 placebo recipients) were randomly chosen from a sample weighted toward participants with low behavioral risk factors for HIV infection. A total of 1725 subjects (98%) were male; because of this preponderance, only male subjects were used in the analyses. A total of 1632 subjects (93%) identified themselves as white.
Risk scores, assigned to each subject during the Vax004 trial, were determined on the basis of self-reported behaviors, including sexual activity and alcohol and drug use, and on the occurrence of sexually transmitted diseases. A standard interviewer-administered questionnaire was used at baseline and every 6 months thereafter.3
FcγR genotyping
DNA was extracted from subjects' peripheral blood mononuclear cells and used for genotyping. For FcγRIIIa, we used a custom TaqMan (Applied Biosystems) assay and standard protocols as recommended by the manufacturer. A total of 772 of these specimens were confirmed by the use of a melt curve–based method as described previously.16,24 For FcγRIIa, 1164 samples were genotyped with a TaqMan assay, and 772 were genotyped by a melt curve-based method; among these specimens, 196 were genotyped by both methods.
Statistical analyses
Cox proportional hazards models were used to evaluate hazard ratios of HIV infection for different genotype groups and for vaccine versus placebo recipients within certain genotype groups. Time-to-infection in the models originated with the time of randomization into the Vax004 trial. These models used estimator II to accommodate the covariate-stratified and outcome-dependent sampling design.25 Wald tests based on the model were used to test whether hazard ratios differed from one and whether the hazard rate was the same across 3 genotype categories. Cumulative HIV-1 incidence curves were plotted for subgroups of subjects, estimated via the Kaplan-Meier method with inverse probability weighting, to account for the sampling design. All P values were 2-sided, with a threshold of .05 used to judge statistical significance.
Results
Distribution of FcγR genotypes
FcγRIIa genotyping was successful in 1740 male subjects, including 1480 vaccinees and 260 placebo recipients. FcγRIIIa genotyping was completed for 1705 male subjects, including 1447 vaccinees and 258 placebo recipients. The distribution of genotypes within these groups was similar to that observed in other cohorts consisting primarily of white subjects (Table 1). No linkage disequilibrium was observed (Χ2 = 2.13; 5 degrees of freedom).
Distribution of FcγRIIa and FcγRIIIa genotypes in vaccinees and placebo recipients
| . | No. vaccinees (%) . | No. placebo recipients (%) . |
|---|---|---|
| FcγRIIa | ||
| RR | 380 (25.7) | 75 (28.9) |
| RH | 754 (50.9) | 126 (48.5) |
| HH | 346 (23.4) | 59 (22.7) |
| Total | 1480 (100) | 260 (100) |
| FcγRIIIa | ||
| FF | 629 (43.4) | 116 (45.0) |
| FV | 644 (44.5) | 108 (41.9) |
| VV | 174 (12.0) | 34 (13.2) |
| Total | 1447 (100) | 258 (100) |
| . | No. vaccinees (%) . | No. placebo recipients (%) . |
|---|---|---|
| FcγRIIa | ||
| RR | 380 (25.7) | 75 (28.9) |
| RH | 754 (50.9) | 126 (48.5) |
| HH | 346 (23.4) | 59 (22.7) |
| Total | 1480 (100) | 260 (100) |
| FcγRIIIa | ||
| FF | 629 (43.4) | 116 (45.0) |
| FV | 644 (44.5) | 108 (41.9) |
| VV | 174 (12.0) | 34 (13.2) |
| Total | 1447 (100) | 258 (100) |
F indicates phenylalanine; FcγR, Fc receptor for IgG; H, histidine; R, arginine; and V, valine.
FcγRIIIa genotype is associated with the rate of HIV infection in vaccines
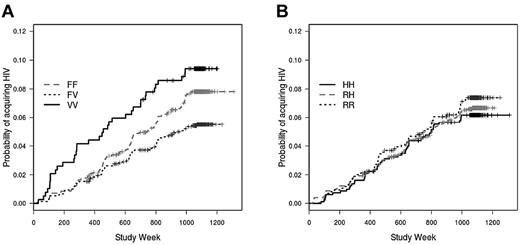
In a Cox model that included race, behavioral risk score, and location, FcγRIIIa genotype was significantly associated with the rate of infection (P = .035; Figure 1A). Unexpectedly, subjects with the heterozygous FV genotype had a lower infection rate (hazard ratio = 0.69; P = .027) compared with the FF (reference) genotype; rates were similar in the VV (hazard ratio = 1.1; P = .60) and FF groups. In a model that included both FcγRIIa and FcγRIIIa, there was again a lower infection rate among vaccinees with the FV genotype compared with the FF genotype (hazard ratio = 0.70; P = .032; not shown). There was no association between FcγRIIa genotype and the rate of HIV infection (P = .42; Figure 1B).
FcγRIIIa genotype, but not FcγRIIa genotype, is associated with acquisition of HIV infection after vaccination with rgp120. Cumulative HIV incidence curves for (A) FcγRIIIa genotype (P = .035) and (B) FcγRIIa genotype are determined by Cox proportional hazard models that are weighted to account for case-cohort design and under sampling of subgroups of uninfected subjects. The curves are unadjusted for race, behavioral risk score, and location.
FcγRIIIa genotype, but not FcγRIIa genotype, is associated with acquisition of HIV infection after vaccination with rgp120. Cumulative HIV incidence curves for (A) FcγRIIIa genotype (P = .035) and (B) FcγRIIa genotype are determined by Cox proportional hazard models that are weighted to account for case-cohort design and under sampling of subgroups of uninfected subjects. The curves are unadjusted for race, behavioral risk score, and location.
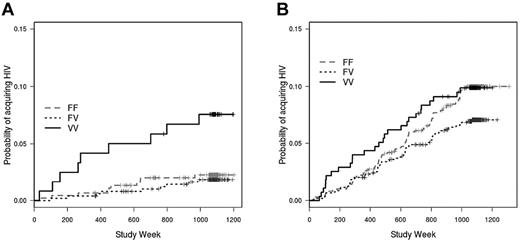
Behavioral risk factors, such as the type and frequency of sexual encounters, and the occurrence of other sexually transmitted infections, are strongly associated with HIV rates in several studies and likely modified the estimate of vaccine efficacy in the Thai RV144 trial.1,26-28 Given the importance of behavioral risk on the incidence of HIV infection, we divided the vaccinated subjects into those with the lowest-risk behaviors (risk score = 0; n = 449) and those with greater-risk behaviors (risk score ≥ 1; n = 998), according to risk scores assigned to subjects during the Vax004 trial.3 In this analysis, we found that among vaccinees with the lowest risk score, the VV genotype was associated with a significantly greater rate of HIV infection compared with the FF genotype (hazard ratio = 2.97; P = .019), with the FV genotype (hazard ratio = 4.12; P = .002), and with the combined FV and FF genotypes (hazard ratio = 3.52; P = .002; Figure 2A). When vaccinees with high risk scores were evaluated, there was a trend toward the FF genotype being associated with a greater infection rate than the FV genotype, although the trend was nonsignificant (hazard ratio = 1.31; P = .09; Figure 2B). The FF genotype was not different from the VV genotype (hazard ratio = 1.02; P = .85) among these high-risk score vaccinees. Thus, the heterozygous advantage of the FV genotype appears to be a result of the opposite impacts of behavioral risk on the associations between infection rate and the VV and FF homozygous genotypes.
Impact of behavioral risk score on association between FcγRIIIa genotype and HIV infection rate in vaccinated subjects. (A) Among rgp120-vaccinated subjects with low behavioral risk scores (risk score = 0), those with the FcγRIIIa VV genotype acquired HIV infection at a greater rate than those with the FV (hazard rate = 4.12; P = .002), FF (hazard ratio = 2.97; P = .019), and combined FF/FV genotypes (hazard rate = 3.52; P = .002). (B) Among vaccinees with high behavioral risk scores (risk score ≥ 1), there is no significant association between FcγRIIIa genotype and infection rate; there is, however, a trend toward a greater infection rate for the FF genotype compared with the FV genotype (hazard ratio = 1.31; P = .09). Risk scores were assigned at enrollment in the Vax004 trial.
Impact of behavioral risk score on association between FcγRIIIa genotype and HIV infection rate in vaccinated subjects. (A) Among rgp120-vaccinated subjects with low behavioral risk scores (risk score = 0), those with the FcγRIIIa VV genotype acquired HIV infection at a greater rate than those with the FV (hazard rate = 4.12; P = .002), FF (hazard ratio = 2.97; P = .019), and combined FF/FV genotypes (hazard rate = 3.52; P = .002). (B) Among vaccinees with high behavioral risk scores (risk score ≥ 1), there is no significant association between FcγRIIIa genotype and infection rate; there is, however, a trend toward a greater infection rate for the FF genotype compared with the FV genotype (hazard ratio = 1.31; P = .09). Risk scores were assigned at enrollment in the Vax004 trial.
FcγR genotypes do not affect HIV infection rates in placebo recipients
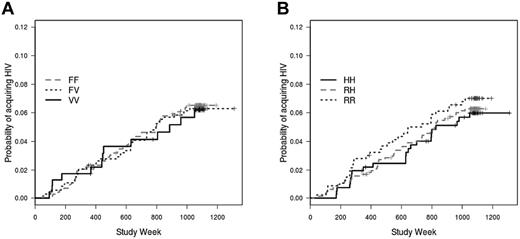
We next evaluated the relationship between the genotypes and infection rates among placebo recipients. Neither the FcγRIIIa (P = .94; Figure 3A) nor the FcγRIIa (P = .92; Figure 3B) genotype was significantly associated with acquisition of HIV infection during the Vax004 trial. Moreover, stratification by risk score had no significant effect on the association between genotypes and infection among placebo recipients (results not shown).
Lack of association between FcγR genotypes and infection rate among placebo recipients. Neither the FcγRIIIa (A) nor FcγRIIa (B) genotype is associated with HIV infection rate among placebo recipients. Cumulative HIV incidence curves are unadjusted.
Lack of association between FcγR genotypes and infection rate among placebo recipients. Neither the FcγRIIIa (A) nor FcγRIIa (B) genotype is associated with HIV infection rate among placebo recipients. Cumulative HIV incidence curves are unadjusted.
FcγR genotypes and vaccine efficacy
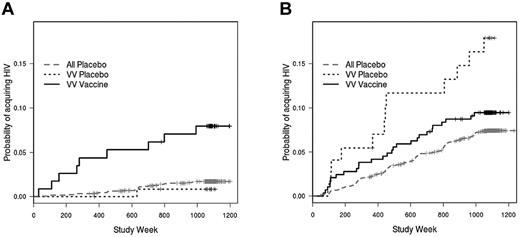
Our observation that the FcγRIIIa genotype influenced the rate of infection among vaccinees but not among placebo recipients suggests that vaccine efficacy (VE) might also differ according to genotype. In initial analyses, neither FcγRIIa nor FcγRIIIa was associated with VE on the basis of interaction tests for VE varying by FcγRIIa and FcγRIIIa (P = .98 and .29, respectively). However, because of the effect of behavioral risk score on infection rate among the VV vaccinees, we proceeded with further analyses stratified by risk score. Indeed, VE within the VV genotype was found to vary significantly by behavioral risk score indicator (interaction test P = .027). Moreover, there was a greater rate of infection among vaccinated VV individuals in the low-risk category than among VV placebo recipients in the low-risk category (hazard ratio = 4.51; P = .17; Figure 4A). This hazard ratio, although high, is not significantly different from 1, possibly reflecting the relatively small number of low-risk placebo recipients with the VV genotype (n = 14). In fact, when low-risk VV vaccinees were compared with all low-risk placebo recipients (n = 98), there was a significant effect of the VV genotype on VE (hazard ratio = 4.72; P = .002; Figure 4A).
Vaccine efficacy among subjects with the FcγRIIIa VV genotype. (A) For subjects with low behavioral risk scores, there is a greater rate of infection among FcγRIIIa VV vaccinees compared with VV placebo recipients (hazard ratio = 4.51; P = .17) or compared with all placebo recipients (hazard ratio = 4.72; P = .002). (B) Among subjects with high behavioral risk scores, VV vaccinees have similar infection rates as VV placebo recipients (hazard ratio = 1.24; P = .64) or all placebo recipients (hazard ratio = 1.27; P = .33).
Vaccine efficacy among subjects with the FcγRIIIa VV genotype. (A) For subjects with low behavioral risk scores, there is a greater rate of infection among FcγRIIIa VV vaccinees compared with VV placebo recipients (hazard ratio = 4.51; P = .17) or compared with all placebo recipients (hazard ratio = 4.72; P = .002). (B) Among subjects with high behavioral risk scores, VV vaccinees have similar infection rates as VV placebo recipients (hazard ratio = 1.24; P = .64) or all placebo recipients (hazard ratio = 1.27; P = .33).
Note that the hazard ratios for the comparison of low-risk VV vaccinees with low-risk VV placebo recipients and for the comparison of low-risk VV vaccinees with all low-risk placebo recipients are nearly identical. Furthermore, there was no effect of genotype on the rate of infection for placebo recipients, and, in particular, low-risk VV placebo recipients did not differ in infection rate from the other low-risk placebo recipients (hazard ratio = 0.67; P = .71). Therefore, we also compared the low-risk VV vaccinees with all low-risk placebo recipients. By expanding the low-risk group to include those with a risk score of ≤ 1 (n = 1092, of whom 164 were placebo recipients), we found that there was a more robust, although lesser, effect of the VV genotype on VE (hazard ratio = 3.15; P = .0001).
Among VVs in the greater-risk category, there was no difference in infection rate between vaccinees and placebo recipients (hazard ratio = 1.24; P = .64; Figure 4B). Similarly, when VV vaccinees in the high-risk category were compared with all placebo recipients, there was no difference in rate of infection (hazard ratio = 1.27; P = .33; Figure 4B). For both low- and high-risk categories, there were no significant effects of the FV or FF genotypes of FcγRIIIa or of any of the FcγRIIa genotypes on VE (not shown).
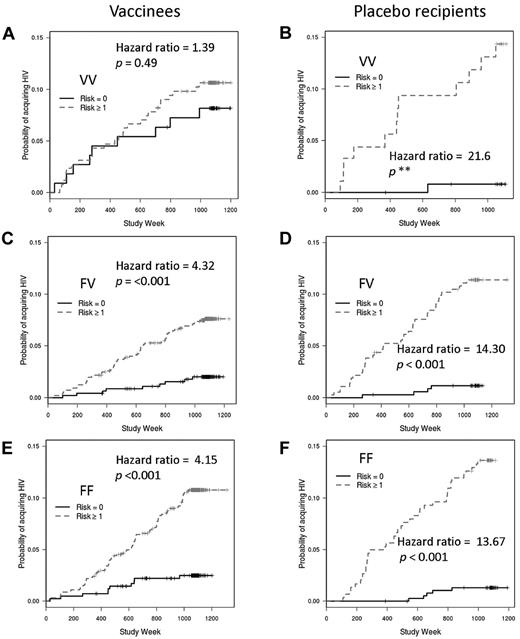
We also explored the interaction between behavioral risk and FcγRIIIa genotype by comparing the influence of risk category on the rates of infection for each genotype. If genotype had no impact on the relationship between risk and infection rate, one would expect greater rates of infection among subjects with greater behavioral risk scores irrespective of genotype. However, vaccinees with the VV genotype had similar infection rates whether they were in the low- or high-risk categories (hazard ratio = 1.39; P = .49; Figure 5A). As expected, among FV and FF vaccinees, there was a very strong association between risk category and infection such that those in the greater risk category (risk score ≥ 1) had the greatest rates of infection (hazard ratio comparing high- to low-risk categories for FV = 4.32; P = < .001; for FF = 4.15; P = < .001; Figure 5C,E). Among recipients of placebo, the expected impact of risk category (that is, greater rate of infection with greater risk category) was seen for all 3 FcγRIIIa genotypes (Figure 5B,D,F). Thus, vaccination appears to impart a high-risk phenotype (with respect to HIV acquisition) to VVs who would otherwise have a low-risk phenotype.
Effects of FcγRIIIa genotypes, vaccine status, and behavioral risk scores on HIV infection rates. Vaccinees with the FcγRIIIa VV genotype have similar infection rates whether in the high behavioral risk or low behavioral risk groups (A). However, behavioral risk is a strong predictor of infection rates for vaccinees with the FV (C) or FF (E) genotypes and for placebo recipients with the VV (B), FV (D), or FF (F) genotypes. Hazard ratios and P values are shown for the comparison between low risk and high risk; the small number of VV placebo recipients results in an unstable hazard ratio and incalculable P value.
Effects of FcγRIIIa genotypes, vaccine status, and behavioral risk scores on HIV infection rates. Vaccinees with the FcγRIIIa VV genotype have similar infection rates whether in the high behavioral risk or low behavioral risk groups (A). However, behavioral risk is a strong predictor of infection rates for vaccinees with the FV (C) or FF (E) genotypes and for placebo recipients with the VV (B), FV (D), or FF (F) genotypes. Hazard ratios and P values are shown for the comparison between low risk and high risk; the small number of VV placebo recipients results in an unstable hazard ratio and incalculable P value.
In the Thai RV144 trial, the estimate of VE was greatest during the first 12 months after vaccination and appeared to decrease subsequently. We divided Vax004 results on the basis of time to seroconversion (≤ 12 months vs > 12 months) and determined VE for each FcγRIIIa genotype within each time period. In these analyses, risk behavior, as well as race and location, were included in the Cox proportional hazard model. There was a trend toward an interaction between FcγRIIIa genotype and vaccination status in predicting infections within the first 12 months (P = .13). In addition, there was a trend toward a greater rate of infection during the first 12 months in VV vaccinees compared with VV placebo recipients (hazard ratio = 2.79; P = .099; Table 2), and VV vaccinees had a significantly greater rate of infection during the first 12 months compared with all placebo recipients (hazard ratio = 2.3; P = .0076). The VV genotype did not significantly modify VE for HIV infection after 12 months. The FV and FF genotypes also did not modify VE during either time period when we compared vaccinees and placebo recipients within the respective genotype groups. Thus, during the first 12 months of the Vax004 trial, VV subjects who were vaccinated with rgp120 may have become infected with HIV at a greater rate than recipients of placebo.
VE by FcγRIIIa genotype among subjects infected within or after 12 months of participation in Vax004
| FcγRIIIa genotype . | ≤ 12 months HR (P) . | > 12 months HR (P) . |
|---|---|---|
| VV | 2.79 (.10) | 1.2 (.68) |
| FV | 0.78 (.45) | 1.07 (.77) |
| FF | 1.06 (.87) | 1.35 (.20) |
| FcγRIIIa genotype . | ≤ 12 months HR (P) . | > 12 months HR (P) . |
|---|---|---|
| VV | 2.79 (.10) | 1.2 (.68) |
| FV | 0.78 (.45) | 1.07 (.77) |
| FF | 1.06 (.87) | 1.35 (.20) |
Cox proportional hazard models include risk behavior score, race, and location. The hazard ratio estimates vaccine efficacy within each FcγRIIIa genotype (eg, VV vaccinees are compared with VV placebo recipients).
HR indicates hazard ratio; F indicates phenylalanine; FcγR, Fc receptor for IgG; V, valine; and VE, vaccine efficiency.
Sera from 269 of the uninfected genotyped subjects in our study had been previously assayed at a single dilution (1:5000) for binding to MN/GNE8 rgp120 by ELISA.4 Using results from the 12-month visit, the mean optical densities (± SD) for the FF, FV, and VV subjects were 0.837 ± 0.62, 0.737 ± 0.58, and 0.827 ± 0.58, respectively; these results were not significantly different from one another (P > .1). Similar results were observed for the 126 subjects measured at the expected peak of their antibody responses (month 12.5). The small number of subjects precluded a meaningful analysis of any interactions between genotype and antibody in predicting infection outcomes.
Discussion
We found an association between FcγRIIIa genotype and the rate of HIV infection after vaccination with rgp120 during the Vax004 trial. This association, wherein individuals with the VV genotype have the greatest rate of infection, is complex and appears to be influenced both by the level of behavioral risk and, possibly, by the timing of infection relative to vaccination. Because a greater rate of infection occurs among VV vaccinees than among placebo recipients, enhanced infection might have resulted from the use of rgp120 vaccine.
Our finding of a relationship between the VV genotype and a greater rate of sexually acquired HIV infection implies a role for Fc-FcγRIIIa interactions in the successful establishment of infection in the setting of vaccine-induced antibody. FcγRIIIa is expressed on the surface of some monocytes, macrophages, and dendritic cells.5 In addition, some γδT cells and the majority of circulating NK cells bear FcγRIIIa, and it is the predominant or exclusive Fc receptor on those cells.29-31 The V isoform has greater avidity to complexes consisting of IgG1, IgG2, and IgG4 than does the F isoform,6 and as a result, binding is greatest for the VV genotype, lowest for the FF genotype, and intermediate for the FV heterozygotes. This difference in binding is most pronounced in the case of IgG2 and IgG4, which are essentially only able to bind to the V isoform.6 Given the greater avidity of IgG for the VV receptor, our results are consistent with a model whereby infectious immune complexes are directly able to preferentially bind, enter, and infect susceptible VV-bearing cells. Mucosal cells that might be infectable in this manner would include macrophages and possibly dendritic cells.32-34 Alternatively, VV-bearing cells might be capable of holding immune complexes and making them more available to T lymphocytes. A third possibility is that cytokine production via FcγRIIIa triggering by immune complexes is more efficient for the VV than for the other forms of the receptor; thus, VV subjects might manifest a greater number of HIV-susceptible T cells. Notably, only a minority of macrophages bear FcγRIIIa,32 and mucosal NK cells are reported to express FcγRIIIa much less frequently than blood NK cells.35 It is unclear whether dendritic cells in relevant mucous membranes express FcγRIIIa. In the presence of local inflammation, however, one might expect an influx of NK cells and macrophages with greater FcγRIIIa expression, which would support a role for such cells in enhancing infection.36 Finally, a role for FcγRIIIa-expressing genital tract epithelial cells remains a possibility.37,38
Risk behavior appears to influence the association between FcγRIIIa genotype and infection rate. Our exploratory analysis suggests that only vaccinated subjects with the lowest behavioral risks are impacted by their genotype. One interpretation of this finding is that infection enhancement because of the VV genotype is unable to add to the already high chance of infection imposed by risky sexual behaviors.
Our results also show a trend toward the VV genotype being associated with a greater rate of infection, independently of behavioral risk, during the first year after enrollment in Vax004. Gilbert et al have found that peak antibody levels, measured in several different assays, rise with each subsequent vaccination until reaching a plateau after the fourth vaccination, which was given at month 12.4 Thus, the population whose infection rate may have been impacted by their FcγRIIIa genotype in our study is likely to have lower antibody levels than subjects infected later in the study. Interestingly, there are several reports of enhancement of HIV-1 infection in vitro by low concentrations of antibodies that neutralize virus or have no effect at greater concentrations.39-42 However, there was no evidence of enhancement of infection during the RV144 trial,43 despite the likelihood that antibody responses in the RV144 trial were lower than in Vax004.44 This finding suggests that the quality of the antibody response, with respect to antiviral function, differed between the 2 trials. FcγRIIIa genotyping results have not been reported for RV144. Finally, we were unable to directly measure the impact of antibody levels on the association between FcγRIIIa genotype and infection rate in Vax004, although in a subset of subjects, antibody levels did not differ by genotype.
Overall, the Vax004 trial demonstrated no vaccine efficacy or enhancement.3 However, in 2 published analyses, greater vaccine-induced antibody levels were associated with lower infection risks in Vax004.4,18 If one population benefited from the vaccine, the lack of efficacy overall might have been because of another population being harmed. Our data indicate that this might have been the case for the approximately 10% of subjects with the VV genotype. However, the 95% confidence interval surrounding VE in Vax004 extended from −17% to 24%, indicating that a small amount of efficacy or enhancement, without its opposite, might have occurred.
In the Step trial, which used an adenovirus 5 gag/pol/nef vaccine, the incidence of HIV was greater in vaccinees compared with placebo recipients among adenovirus 5–seropositive and uncircumcised men. We do not anticipate similarities between the Step and Vax004 trials with respect to potential mechanisms enhancing infection risk, because the Step trial generated primarily CD8+ T-cell responses and Vax004 primarily antibody responses against HIV.4,45,46 Because Vax004 vaccinees almost uniformly developed anti-gp120 antibody responses and because the major ligand for FcγRIIIa is IgG, our results raise the possibility that vaccine-induced antibody might enhance the risk of acquiring sexually transmitted HIV infection. ADE occurs in vitro with several viruses, and in the case of dengue virus infection, likely results in increased disease severity in humans.19-23 Vaccine-induced responses to an early-generation measles vaccine and to a respiratory syncytial virus vaccine underlie atypical disease manifestations after infection with those viruses.47,48 To our knowledge, our findings provide the first evidence, albeit indirect, of in vivo, vaccine-elicited ADE that increases the risk of infection in humans.
Finally, the robustness of our results is subject to the sample size available and to the multiple comparisons performed and must be considered hypothesis generating. Analyses of other large vaccine trials, additional analyses of RV144, and the results of in vitro modeling of genotype-associated enhancement of infection may provide further insights into the role of FcγRIIIa in vaccine efficacy and into whether or not FcγRIIIa genotype should be considered an eligibility criterion for participation in future trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the contributions of Vax004 study participants.
This research was supported by grant R21AI073147 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: D.N.F. conceived and designed the research, contributed to the analyses, and drafted and edited the paper; E.E.G. performed statistical analyses and assisted in editing the paper; A.W. and T.B.P. provided technologic assistance and contributed to drafting the paper; and G.L. contributed to the design of the research and assisted in editing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald N. Forthal, Division of Infectious Diseases, Department of Medicine, University of California, Irvine School of Medicine, 3044 Hewitt Hall, UC Irvine, Irvine, CA 92697; e-mail: dnfortha@uci.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal