Abstract
Graft-versus-host disease (GVHD) remains the most common cause of nonrelapse-related morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although T-cell depletion and intensive immunosuppression are effective in the control of GVHD, they are often associated with higher rates of infection and tumor recurrence. In this study, we showed that heparan sulfate (HS), an extracellular matrix component, can activate Toll-like receptor 4 on dendritic cells in vitro, leading to the enhancement of dendritic cell maturation and alloreactive T-cell responses. We further demonstrated in vivo that serum HS levels were acutely elevated at the onset of clinical GVHD in mice after allo-HSCT. Treatment with the serine protease inhibitor α1-antitrypsin decreased serum levels of HS, leading to a reduction in alloreactive T-cell responses and GVHD severity. Conversely, an HS mimetic that increased serum HS levels accelerated GVHD. In addition, in patients undergoing allo-HSCT for hematologic malignancies, serum HS levels were elevated and correlated with the severity of GVHD. These results identify a critical role for HS in promoting acute GVHD after allo-HSCT, and they suggest that modulation of HS release may have therapeutic potential for the control of clinical GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for many types of hematologic malignancies and nonmalignant hematologic diseases.1 However, graft-versus-host disease (GVHD) remains a prevalent and serious side effect that limits the effectiveness of this therapy.2,3 Although T-cell depletion (TCD) of the bone marrow graft results in decreased rates of GVHD,4,5 it is associated with general immunodeficiency that predisposes recipients to higher rates of viral and fungal infections,6 as well as increased tumor recurrence rates.7 In fact, some level of GVHD may be beneficial to the recipient by being associated with a more robust graft-versus-tumor response as demonstrated by lower tumor recurrence rates in these patients.8
Innate immunity is the rapid response system by which a host can recognize and respond to infection or tissue injury. The rapidity of the innate response is because of fixed pattern recognition receptors that are naturally abundant and poised for immediate response. Toll-like receptors (TLRs), the best characterized family of pattern recognition receptors, were originally characterized for their ability to respond to exogenous pathogen-associated molecular patterns (PAMPs) that include bacterial lipopolysaccharide (LPS), bacterial diacylated and triacylated lipopeptides, bacterial flagellin, bacterial and viral unmethylated CpG-containing DNA motifs, and viral single- and double-stranded RNA.9 In addition to PAMPs, TLRs also recognize endogenous damage-associated molecular patterns (DAMPs). Examples include proteins such as heat-shock protein (Hsp) 60, Hsp70, surfactant protein A, high-mobility group box 1 (HMGB1), fibrinogen and fibronectin, as well as polysaccharides such as hyaluronan and heparan sulfate (HS).10,11
It has become increasingly apparent that TLRs play a critical role in shaping effective adaptive immune responses in a variety of conditions, such as infection, cancer, and autoimmunity.12,13 It also has been shown that TLR4 and MyD88 deficiencies are protective against acute rejection in the setting of solid organ transplantation.14-16 Similarly, stimulation of TLR9 with CpG oligodeoxynucleotides markedly accelerates GVHD lethality,17 suggesting a role for TLR pathways in modulating GVHD. Because the onset of GVHD usually occurs in the absence of obvious exogenous stimuli such as bacterial or viral infections, we sought to investigate the role of endogenous TLR agonists in the development of GVHD.
We first found that HS, a ubiquitous component of the extracellular matrix, was a potent stimulator of T-cell alloreactivity in vitro. The stimulatory effect of HS was dependent on an intact TLR4 pathway in dendritic cells (DCs), but not in alloreactive T cells, by promoting DC maturation and function. We next observed that serum levels of HS were highly elevated at the onset of clinical symptom of GVHD in an experimental model of allo-HSCT. Suppression of HS release by the serum protease inhibitor α1-antitrypsin (A1AT) decreased the levels of serum HS, inhibited the activation of donor-derived T cells in vivo, and resulted in a significant improvement in GVHD and survival. Conversely, we demonstrate that increasing serum levels of HS during GVHD using an HS mimetic increased donor T-cell proliferation in vivo and GVHD severity. In human recipients of allo-HSCT, we found that serum HS levels were increased and correlated to the severity of GVHD. These studies demonstrate that HS can promote the alloreactive T-cell response and increase the severity of GVHD, and they suggest that strategies to block HS release may be an effective strategy in the prevention of GVHD.
Methods
Mice
BALB/c mice were purchased from the National Cancer Institute. B10.D2 and TLR4−/− BALB/c mice were purchased from The Jackson Laboratory. MyD88−/− mice were kindly provided by Dr Shizuo Akira (Osaka University, Osaka, Japan) and have been backcrossed for greater than 10 generations onto the BALB/c background. Donor mice were males between 8 and 12 weeks of age, and recipient mice were males between 12 and 16 weeks of age (∼ 22-26 g). All experimental procedures involving the use of mice were done in accordance with protocols approved by the Animal Care and Use Committee of Duke University.
Reagents and cell lines
The following reagents were used: LPS from Escherichia coli O111:B4 (List Biological Laboratories), endotoxin-free Pam3CSK4 (InvivoGen), bovine kidney heparan sulfate (Seikagaku), Hyaluronan Select HA 150K (S-0201; Sigma-Aldrich), fibronectin (F-2006; Sigma-Aldrich), fibrinogen (Hyphen BioMed), HMGB1 (Abnova), Hsp70 (Assay Designs), C12-IE-DAP and L18-MDP (InvivoGen), protamine sulfate (Sigma-Aldrich), and murine GM-CSF (R&D Systems). Sonicated hyaluronan, a gift from Dr Stavros Garantziotis (National Institute of Environmental Health Sciences, Research Triangle Park, NC) was produced as described previously.18
Human epithelial kidney (HEK) 293 cell lines coexpressing human CD14, MD2, and TLR4 or TLR2 were a kind gift from Dr Michael Fessler (National Institute of Environmental Health Sciences). Some assays were performed in the presence of polymyxin B (Sigma-Aldrich) or after preincubation with heparinase III from Flavobacterium heparinum (Sigma-Aldrich). One unit of heparinase was incubated with 50 μg of HS or 50 ng LPS in 25 μL culture medium at 32°C for 6 hours.
allo-HSCT
For allo-HSCT, wild-type (WT) and TLR4−/− BALB/c mice received myeloablative total-body irradiation (8.5 Gy) followed by intravenous infusion of 1 × 107 B10.D2 or BALB/c T cell–depleted bone marrow (TCD-BM). Bone marrow was prepared as described previously,19 and the T cells were depleted using Thy1.2 (Invitrogen) or CD5 (Miltenyi Biotec)–conjugated magnetic beads according to the manufacturers' instructions. To induce GVHD, 5 × 106 lymph node cells from inguinal, axillary, cervical, and mesenteric lymph nodes of B10.D2 or BALB/c mice where injected intravenously in addition to TCD-BM. In another model of acute GVHD, 5 × 106 lymph node cells and 1 × 107 TCD-BM from C3H.SW were transferred to C57BL/6 recipients irradiated with 10 Gy. HSCT recipients of these 2 GVHD models then received either intraperitoneal injections of 2 mg of A1AT (Aralast; Baxter) resuspended in 200 μL of sterile phosphate-buffered saline (PBS), subcutaneous injections of 20 mg/kg PG545 (Progen Pharmaceuticals Limited) in 200 μL of PBS, or 200 μL PBS alone at the indicated intervals.
Assessment of GVHD
GVHD severity was assessed using the previously described clinical scoring system that accounts for 5 parameters: weight loss, fur texture, skin integrity, hunching posture, and activity.20 End points for survival were death, moribund status, or weight loss > 30%. Histologic analysis of GVHD was performed on full-thickness ear tissue. After fixation in fresh neutral buffered formalin for 24 hours, ear tissue was routinely processed and embedded in paraffin. The 5-μm-thick sections were stained with hematoxylin and eosin. These de-identified slides were evaluated by a single pathologist (DMC) blinded to experimental groups and graded in a semiquantitative manner on the basis of dermal fibrosis, fat loss, inflammation, epidermal interface changes, and follicular dropout (0-2 for each category).21
Antibodies and flow cytometry
Anti-CD40 (HM40-3), anti-CD80 (16-10A1), anti-Thy1.1 (OX-7), anti-Ly5.1 (A20), anti–IFN-γ (XMG1.2), rat IgG1 isotype (R3-34), and the BrdU flow kit (FITC-labeled) were from BD Biosciences. Intracellular IFN-γ staining was performed as described previously.22 For in vivo BrdU labeling, mice were injected with 50 μg of BrdU/g intraperitoneally 1 hour before analysis. Collection of flow cytometric data was acquired using an FACSCanto flow cytometer (BD Biosciences), and events were analyzed using FlowJo Version 9.3 software (TreeStar).
T-cell proliferation assay
Cytokine analysis
Cell culture supernatants were obtained from DC cultures or T-cell proliferation assays and assayed for IL-6, IL-12, and IFN-γ by ELISA (BD Biosciences) according to the manufacturer's standard protocols. HEK 293 supernatants were tested for human IL-8 by ELISA (BioLegend).
Luciferase reporter assay
Luciferase activity was measured using the Dual-Luciferase Reporter Assay (Promega) according to the manufacturer's recommended protocol. Luminescence was measured using an LMax luminometer (Molecular Devices).
HS ELISA
Serum samples were assayed for HS concentration by ELISA (Amsbio) according to the manufacturer's recommended protocol.
HS levels also were measured in patients undergoing allo-HSCT under an institution-sponsored institutional review board (Pro00031607). Patient data were obtained by chart review. Serum samples were collected from patients at various time points relative to GVHD and assayed for HS levels by ELISA as described in this section.
TCR-tg adoptive transfer
Lymph node (LN) cells purified from 4C–T-cell receptor transgenic (TCR-tg) mice on the C57BL/6-Ly5.1 background were labeled with carboxy fluorescein succinimidyl ester (CFSE; Invitrogen) as described previously.22 BALB/c recipients of C57BL/6 HSCTs (107 TCD-C57BL/6 BM and 106 C57BL/6 LN cells) were intravenously injected with 2 × 106 CFSE-labeled LN cells 14 days after transplant. Three days later, mice were killed, their livers were harvested, and intrahepatic lymphocytes were purified after mechanical disruption on a discontinuous Ficoll (Sigma-Aldrich) gradient.
Statistical analysis
Results are expressed as mean ± SEM. Comparison between groups was performed by Kruskal-Wallis and Student t test for continuous variables, Fisher exact test for categorical variables, and log rank test for survival data. All statistical analyses were performed using Prism Version 5.0 software (GraphPad Software). Differences are reported to be significant with P ≤ .05.
Results
HS promotes alloreactive T-cell proliferation by stimulating TLR4 on DCs
To identify endogenous innate immune activators with significant contribution to the alloimmune response, we first examined various DAMPs that have been implicated in stimulating TLR pathways in promoting alloreactive T-cell responses. Purified T cells from C57BL/6 mice were stimulated with bone marrow–derived DCs from BALB/c mice, and their proliferation was measured by the incorporation of [3H]thymidine. DAMPs tested included the proteins fibronectin, fibrinogen, Hsp70, and HMGB1 and the glucosaminoglycans HS and hyaluronan. For comparison, we also tested 2 well-described PAMPs, LPS and a synthetic tripalmitoylated lipopeptide Pam3CSK4, ligands of the TLR4 homodimer and the TLR1/2 heterodimer, respectively. As nucleotide-binding domain, leucine-rich repeat–containing receptors (NLRs) are another family of innate immune receptors, we further tested ligands of NLR1 (C12-iE-DAP) and NLR2 (L18-MDP). We found that among DAMPs, only HS and Hsp70 significantly increased alloreactive T-cell proliferation compared with media alone (Figure 1A). The stimulation by HS was comparable with that achieved by PAMPs such as LPS and Pam3CSK4. Neither of the NLR ligands tested produced a significant increase in alloreactive T-cell proliferation.
HS is a potent stimulator of alloreactive T-cell responses through the TLR4- and MyD88-dependent activation of DCs. (A) TLR and NLR agonists were assayed in allogeneic T-cell proliferation assay between purified T cells (2 × 105/well) from C57BL/6 mice and bone marrow–derived BALB/c DCs (2.5 × 104/well). Cells were cocultured either alone (media) or in the presence of LPS (100 ng/mL), Pam3CSK4 (2 μg/mL), hyaluronan (HA; 100 μg/mL), sonicated-HA (sHA; 100 μg/mL), fibronectin (FN; 100 μg/mL), fibrinogen (Fbn; 100 μg/mL), HS (100 μg/mL), Hsp70 (5 μg/mL), HMGB1 (1 μg/mL), C12-iE-DAP (1 μg/mL), or L18-MDP (1 μg/mL) for 72 hours and then pulsed [3H]thymidine for 16 hours. Proliferation was determined by 3H incorporation and results are expressed as cpm ± SEM. Baseline alloreactivity is indicated by the dotted line (*P < .05 compared with media alone). (B) Proliferation performed as described in panel A ± the addition of the LPS inhibitor polymyxin B (PMB; 10 μg/mL; *P < .05). (C) Proliferation assay performed as described in panel A with purified responder T cells (R) from either WT (+) or MyD88−/− (−) C57BL/6 mice were cocultured with DC stimulators (S) from either WT (+) or MyD88−/− (−) BALB/c mice (*P < .05 compared with media alone in S+/R+ group. (D-E) Analysis of proliferation and IFN-γ production in proliferation assays performed as described in panel A using WT, TLR4−/−, and MyD88−/− BALB/c DCs as stimulators and purified C57BL/6 T cells as responders (*P < .05). Results are representative of 3 independent experiments.
HS is a potent stimulator of alloreactive T-cell responses through the TLR4- and MyD88-dependent activation of DCs. (A) TLR and NLR agonists were assayed in allogeneic T-cell proliferation assay between purified T cells (2 × 105/well) from C57BL/6 mice and bone marrow–derived BALB/c DCs (2.5 × 104/well). Cells were cocultured either alone (media) or in the presence of LPS (100 ng/mL), Pam3CSK4 (2 μg/mL), hyaluronan (HA; 100 μg/mL), sonicated-HA (sHA; 100 μg/mL), fibronectin (FN; 100 μg/mL), fibrinogen (Fbn; 100 μg/mL), HS (100 μg/mL), Hsp70 (5 μg/mL), HMGB1 (1 μg/mL), C12-iE-DAP (1 μg/mL), or L18-MDP (1 μg/mL) for 72 hours and then pulsed [3H]thymidine for 16 hours. Proliferation was determined by 3H incorporation and results are expressed as cpm ± SEM. Baseline alloreactivity is indicated by the dotted line (*P < .05 compared with media alone). (B) Proliferation performed as described in panel A ± the addition of the LPS inhibitor polymyxin B (PMB; 10 μg/mL; *P < .05). (C) Proliferation assay performed as described in panel A with purified responder T cells (R) from either WT (+) or MyD88−/− (−) C57BL/6 mice were cocultured with DC stimulators (S) from either WT (+) or MyD88−/− (−) BALB/c mice (*P < .05 compared with media alone in S+/R+ group. (D-E) Analysis of proliferation and IFN-γ production in proliferation assays performed as described in panel A using WT, TLR4−/−, and MyD88−/− BALB/c DCs as stimulators and purified C57BL/6 T cells as responders (*P < .05). Results are representative of 3 independent experiments.
To exclude the possibility of LPS contamination as a cause for the stimulatory effect of HS, we performed the proliferation assay with HS in the presence or absence of the LPS inhibitor polymyxin B (PMB; Figure 1B). PMB caused a significant decrease in LPS-induced proliferation but not in HS-induced proliferation, indicating that LPS contamination was not responsible for the increase in responder T-cell proliferation observed with HS treatment.
We next investigated whether HS enhanced proliferation of allogeneic T cells was because of the stimulation of TLRs on DCs or T cells. Because MyD88 is a common adaptor protein involved in the signal transduction of all TLRs with the exception of TLR3,9 HS was tested against DCs or T cells that were deficient for MyD88. For this purpose, T cells from WT or MyD88−/− C57BL/6 mice were stimulated with DCs from WT or MyD88−/− BALB/c mice in an allogeneic proliferation assay in the presence of HS, LPS, Pam3CSK4, or media alone. As shown in Figure 1C, the lack of MyD88 in DCs, but not in allogeneic T cells, abolished the enhanced proliferation by HS, which was similar to those found with LPS and Pam3CSK4, with the exception that LPS still produced a reduced, but significant, increase in proliferation with MyD88−/− DCs as stimulators. These results suggest that HS stimulates TLRs on DCs, but not T cells, to enhance T-cell proliferation.
Because HS has been shown as a TLR4 ligand,23,24 we tested whether the absence of TLR4 expression on stimulating DCs would reduce HS-induced alloreactive T-cell proliferation equivalent to MyD88 deficiency. Purified T cells from C57BL/6 mice were cocultured with irradiated WT, TLR4−/−, and MyD88−/− BALB/c DCs, and proliferation was measured by the incorporation of [3H]thymidine. Indeed, HS was not able to increase the proliferation of allogeneic T cells above baseline (Figure 1D) or increase IFN-γ production (Figure 1E) when TLR4−/− DCs were used, similarly to when MyD88−/− DCs were used. These results indicate that an intact TLR4-MyD88 pathway in DCs, but not in T cells, is necessary for HS to promote the alloreactive T-cell response.
HS stimulates TLR4-dependent DC maturation and function
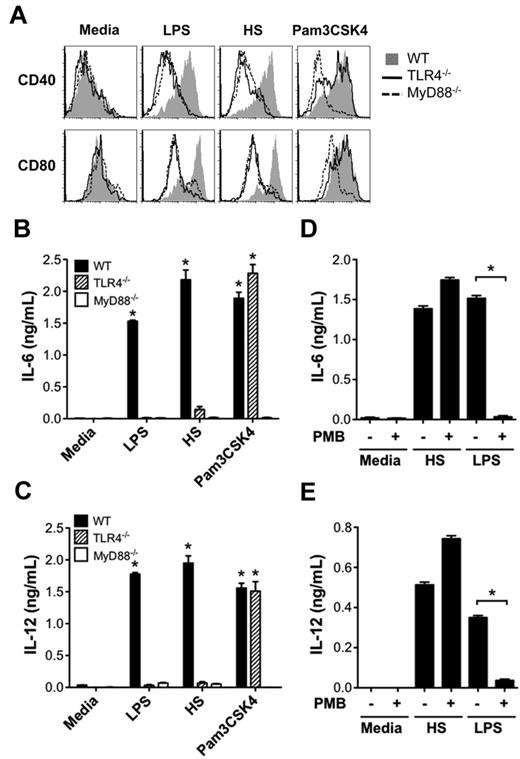
We next investigated how activation of DCs by HS promoted the alloreactive T-cell response. Because DC maturation and production of proinflammatory cytokines are key initial events in triggering adaptive immune responses, we tested the ability of HS to up-regulate DC expression of costimulatory molecules CD40 and CD80 and proinflammatory cytokines IL-6 and IL-12. We found that similarly to LPS, HS caused the up-regulation of CD40 and CD80 on WT DCs, but not on TLR4−/− or MyD88−/− DCs (Figure 2A). In comparison, Pam3CSK4 (TLR1/2 ligand) was able to up-regulate CD40 and CD80 on WT and TLR4−/− DCs, but not on MyD88−/− DCs. Similarly, HS stimulated DCs to produce proinflammatory cytokines IL-6 and IL-12 from WT DCs, but not from TLR4−/− or MyD88−/− DCs (Figure 2B-C) that was not inhibited by PMB (Figure 2D-E).
HS promotes DC maturation and production of proinflammatory cytokines via the TLR4-MyD88 pathway. (A) WT, TLR4−/−, or MyD88−/− BALB/c DCs (2 × 105/well) were stimulated with LPS (100 ng/mL), HS (100 μg/mL), or Pam3CSK4 (2 μg/mL), or left unstimulated (media) for 24 hours and then measured for surface expression of costimulatory molecules CD40 and CD80 by FACS analysis. (B-C) WT, MyD88−/−, and TLR4−/− BALB/c cultured DCs were cocultured with media alone, LPS, HS, or Pam3CSK4 as described in panel A, and culture supernatants were tested for IL-6 (B) and IL-12 (C) by ELISA. Data are representative of 3 independent experiments (*P < .05 compared with media alone). (D-E) Assay of DC production of IL-6 and IL-12 ± the addition of the LPS inhibitor PMB (10 μg/mL) after stimulation with media, HS, or LPS (*P < .05).
HS promotes DC maturation and production of proinflammatory cytokines via the TLR4-MyD88 pathway. (A) WT, TLR4−/−, or MyD88−/− BALB/c DCs (2 × 105/well) were stimulated with LPS (100 ng/mL), HS (100 μg/mL), or Pam3CSK4 (2 μg/mL), or left unstimulated (media) for 24 hours and then measured for surface expression of costimulatory molecules CD40 and CD80 by FACS analysis. (B-C) WT, MyD88−/−, and TLR4−/− BALB/c cultured DCs were cocultured with media alone, LPS, HS, or Pam3CSK4 as described in panel A, and culture supernatants were tested for IL-6 (B) and IL-12 (C) by ELISA. Data are representative of 3 independent experiments (*P < .05 compared with media alone). (D-E) Assay of DC production of IL-6 and IL-12 ± the addition of the LPS inhibitor PMB (10 μg/mL) after stimulation with media, HS, or LPS (*P < .05).
TRIF is a well-described intracellular signal transduction adaptor molecule involved in MyD88-independent TLR4 signal transduction.25 We therefore tested HS stimulation of IL-6 production from WT, TLR4−/−, MyD88−/−, and TRIF−/− C57BL/6 DCs. We found that although MyD88 deficiency prevented the majority of the HS-induced IL-6 production, TRIF deficiency had a smaller, but significant, effect on the production of IL-6 on stimulation with HS and LPS, but not CpG (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Because a heterodimer of different TLRs is required for some PAMPs, we sought to determine whether TLR4 was sufficient for HS activity. For this purpose, we tested the ability of HS to activate HEK cell lines stably transfected to express human TLR4 or human TLR2 in addition to the coreceptors CD14 and MD2. These cell lines were transfected with a NF-κB promoter–driven firefly luciferase reporter plasmid along with a thymidine kinase promoter–driven Renilla luciferase reporter plasmid as a transfection control. The ratio of luminescence produced by firefly luciferase to Renilla luciferase was then measured in response to media alone, LPS, HS, and Pam3CSK4. We also measured the production of IL-8, which is downstream of NF-κB activation, by ELISA. Similar to LPS, we found that HS caused NF-κB activation (supplemental Figure 2A), as well as IL-8 expression (supplemental Figure 2B) in TLR4-expressing cell lines. Thus, TLR4 is sufficient for HS-induced NF-κB activation and IL-8 production.
To control for potential LPS contamination, PMB was added to the cultures. Treatment with PMB inhibited IL-8 expression resulting from LPS treatment, but not HS treatment (supplemental Figure 2C). To demonstrate a direct role of HS, we next tested HS pretreated with heparanase. Heparanase significantly reduced HS stimulation, but not LPS stimulation, demonstrating that HS induced IL-8 expression was specific to HS (supplemental Figure 2D).
Serum levels of HS are elevated at the onset of GVHD in a murine model of allo-HSCT
To investigate the in vivo relevance of HS in the setting of alloimmunity, serum levels of HS were tested in a mouse model of GVHD in the setting of allo-HSCT (Figure 3A). In this model, lethally irradiated BALB/c mice were transplanted with 1 × 107 TCD-BM and 5 × 106 lymphocytes (LCs) from B10.D2 mice (allo-BM + LC). Control mice received B10.D2 TCD-BM only (allo-BM only) or syngeneic BALB/c TCD-BM and 5 × 106 BALB/c LCs (Syn-BM + LC). Serum HS levels were significantly elevated in the recipients of allo-BM + LC on posttransplant days 9 and 14, and they returned to baseline by posttransplant day 22. Notably, the increase in HS occurred before the onset of GVHD symptoms. To determine whether the in vivo concentrations of HS were sufficient to cause DC activation, we tested for the production of IL-6 by cultured DCs in response to a range of HS concentrations (Figure 3B). Under these conditions, we determined that maximal response was achieved by HS concentrations of 12.5 μg/mL and that the half-maximal effective concentration of HS was approximately 4 μg/mL.
Serum HS is highly elevated at the onset of GVHD. Lethally irradiated BALB/c recipients received either 1 × 107 B10.D2 TCD-BM only (allo-BM), 1 × 107 B10.D2 TCD-BM and 5 × 106 B10.D2 LCs (allo-BM + LC), or 1 × 107 BALB/c TCD-BM and 5 × 106 BALB/c LCs (Syn-BM + LC). (A) After transplantation, serum HS concentrations were determined by ELISA at the indicated time points; n = 2-5 samples per time point (*P < .05 comparing allo-BM + LC and allo-BM at the indicated time point. (B) To determine the half-maximal effective concentration of HS on DC stimulation, BALB/c DCs (2 × 105/well) were cultured for 24 hours with differing concentrations of HS in triplicate, and IL-6 production was tested by ELISA. Results are representative of 3 independent experiments.
Serum HS is highly elevated at the onset of GVHD. Lethally irradiated BALB/c recipients received either 1 × 107 B10.D2 TCD-BM only (allo-BM), 1 × 107 B10.D2 TCD-BM and 5 × 106 B10.D2 LCs (allo-BM + LC), or 1 × 107 BALB/c TCD-BM and 5 × 106 BALB/c LCs (Syn-BM + LC). (A) After transplantation, serum HS concentrations were determined by ELISA at the indicated time points; n = 2-5 samples per time point (*P < .05 comparing allo-BM + LC and allo-BM at the indicated time point. (B) To determine the half-maximal effective concentration of HS on DC stimulation, BALB/c DCs (2 × 105/well) were cultured for 24 hours with differing concentrations of HS in triplicate, and IL-6 production was tested by ELISA. Results are representative of 3 independent experiments.
A1AT reduces serum HS levels and improves the outcome of GVHD in a TLR4-dependent manner
We next studied the significance of HS elevation in the development of GVHD. A1AT is a potent serum protease inhibitor used in patients with α1-antitypsin deficiency to protect against neutrophil elastase-induced lung injury.26 It has been shown previously that intravenous treatment of mice with elastase, a protease that cleaves HS-containing proteoglycans within the extracellular matrix, causes a systemic inflammatory response syndrome that is similar to that occurring when HS is injected directly.24 Thus, we sought to determine whether the administration of A1AT would reduce serum HS levels after allo-HSCT. A1AT (2 mg) was administered to allo-BM + LC recipients by intraperitoneal injection every 3 days beginning 1 day before transplant, as described previously for the use of A1AT therapy for tolerance induction in the setting of pancreatic islet transplantation.27 Compared with injections of PBS alone, A1AT-treated recipients had significantly lower serum HS levels at days 9 and 14 after HSCT (Figure 4A). In the comparison of survival between the A1AT-treated and PBS-treated recipients, A1AT therapy resulted in significantly longer survival compared with PBS-treated controls (MST 48.5 vs 28.0 days; P < .001; Figure 4B).
A1AT decreases serum HS levels and improves the outcome of GVHD after allo-HSCT. (A) Serum HS concentrations were determined by ELISA at the indicated time points after allo-HSCT (B10.D2→BALB/c; 1 × 107 B10.D2 TCD-BM and 5 × 106 B10.D2 LC) treated with A1AT (2 mg) or PBS every 3 days by intraperitoneal injection, starting 1 day before transplantation; n = 3 per data point (*P < .05). Survival (B) and GVHD clinical score (C) of allo-BM only (n = 5) or allo-BM + LC treated with A1AT (n = 8) or PBS (n = 5). Data are from 1 of 2 independent experiments with identical results. GVHD pathology score (D) and representative H&E histology (E) of BALB/c recipients of B10.D2 (allo) TCD-BM + LC treated with PBS or with A1AT (n = 6 per group; bar denotes 100 μm (*P = .05). The micrographs were taken from H&E sections (200× magnification) using the 20× PlanApochromatic objective with an Olympus Vanox-AHBS-3 microscope. The camera used is Olympus DP-70 with its own acquisition software. (F) Survival of lethally irradiated C57BL/6 recipients of 1 × 107 C3H.SW TCD-BM (allo-BM) and 5 × 106 C3H.SW LC administered A1AT (n = 10) or PBS (n = 9). (G) Improvement in GVHD survival by A1AT is dependent on host TLR4 expression. Survival of BALB/c recipients of allo-BM + LC from B10.D2 donors (n = 11) and TLR4−/− BALB/c recipients of allo-BM + LC from B10.D2 donors administered A1AT (n = 8) or PBS (n = 14) every 3 days by intraperitoneal injection, starting 1 day before transplantation.
A1AT decreases serum HS levels and improves the outcome of GVHD after allo-HSCT. (A) Serum HS concentrations were determined by ELISA at the indicated time points after allo-HSCT (B10.D2→BALB/c; 1 × 107 B10.D2 TCD-BM and 5 × 106 B10.D2 LC) treated with A1AT (2 mg) or PBS every 3 days by intraperitoneal injection, starting 1 day before transplantation; n = 3 per data point (*P < .05). Survival (B) and GVHD clinical score (C) of allo-BM only (n = 5) or allo-BM + LC treated with A1AT (n = 8) or PBS (n = 5). Data are from 1 of 2 independent experiments with identical results. GVHD pathology score (D) and representative H&E histology (E) of BALB/c recipients of B10.D2 (allo) TCD-BM + LC treated with PBS or with A1AT (n = 6 per group; bar denotes 100 μm (*P = .05). The micrographs were taken from H&E sections (200× magnification) using the 20× PlanApochromatic objective with an Olympus Vanox-AHBS-3 microscope. The camera used is Olympus DP-70 with its own acquisition software. (F) Survival of lethally irradiated C57BL/6 recipients of 1 × 107 C3H.SW TCD-BM (allo-BM) and 5 × 106 C3H.SW LC administered A1AT (n = 10) or PBS (n = 9). (G) Improvement in GVHD survival by A1AT is dependent on host TLR4 expression. Survival of BALB/c recipients of allo-BM + LC from B10.D2 donors (n = 11) and TLR4−/− BALB/c recipients of allo-BM + LC from B10.D2 donors administered A1AT (n = 8) or PBS (n = 14) every 3 days by intraperitoneal injection, starting 1 day before transplantation.
Clinical scores for GVHD were compared between experimental groups using a 10-point scale.20 All groups had an elevation in clinical score between 3 and 7 days after transplantation related to radiation exposure (Figure 4C). The elevation in clinical score quickly returned to the baseline thereafter in the cohort that received allogeneic TCD-BM alone. However, the cohort that received the allogeneic TCD-BM and LCs (allo-BM + LC) had a progressive increase in clinical score beginning 20 days after transplantation until they reached their clinical end point (death or 30% weight loss). The allo-BM + LC recipients treated with A1AT had a significantly lower clinical score curve (P = .03).
Severity of GVHD also was examined by histology. Ear skin was obtained from allo-BM + LC mice at 3 weeks posttransplant from A1AT- or PBS-treated recipients and scored for GVHD severity. A1AT-treated mice demonstrated significantly lower GVHD pathology scores (Figures 4D-E).
To investigate whether the improved survival by A1AT administration in the setting of allo-HSCT may be because of the suppression of alloreactive T-cell responses, donor T cells were tested for in vivo proliferation by BrdU incorporation and for function by IFN-γ production after allo-HSCT. To monitor the behavior of alloreactive T cells in vivo, we used allogeneic TCD-BM and LCs isolated from Thy1.1+ B10.D2 donor mice. Recipients were pulsed with BrdU 6 days after transplantation, and their splenocytes were FACS-analyzed 1 hour later. We found that the BrdU incorporation and IFN-γ production by donor Thy1.1+ T cells were significantly reduced in recipient mice treated with A1AT (supplemental Figure 3A-C).
To further support the survival benefit obtained from A1AT therapy, a second GVHD model was performed. Lethally irradiated C57BL/6 recipients were transplanted with 107 C3H.SW TCD-BM and 5 × 106 C3H.SW LCs. Again, A1AT-treated recipients demonstrated a significant survival benefit compared with the PBS-treated group (P = .032; Figure 4F).
Based on our in vitro results, the contribution of HS toward allospecific T-cell activation is TLR4-dependent. We compared the survival of WT BALB/c recipients of B10.D2 TCD-BM and LCs to TLR4−/− BALB/c recipients that either received A1AT injections or PBS control injection. Compared with WT BALB/c recipients, TLR4−/− recipients had a significantly longer survival (57 vs 29 days, P < .001). However, no further survival benefit was observed in TLR4−/− recipients treated with A1ATcompared with the PBS-treated TLR4−/− mice (P = .30; Figure 4G), suggesting the effect of A1AT in vivo also is dependent on TLR4.
HS mimetic PG545 increases serum HS levels and exacerbates GVHD
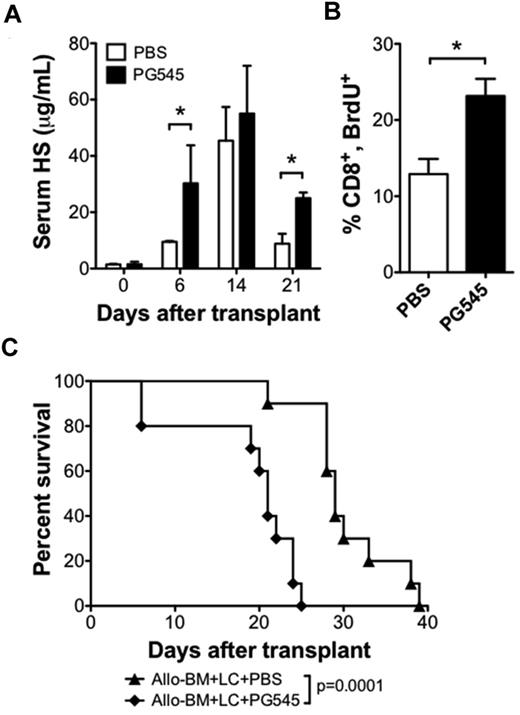
We next sought to test whether increasing serum levels of HS could increase the alloreactive T-cell response and accelerate GVHD. For this purpose, we used the HS mimetic PG545 (Progen Pharmaceuticals) that may function as a competitive inhibitor of heparanase.28 We administered 20 mg/kg PG545 once per week by subcutaneous injection beginning 1 day before transplant. PG545 therapy resulted in higher posttransplant serum HS levels (Figure 5A). Analysis of BrdU uptake on posttransplant day 6 revealed that a higher percentage of proliferating donor CD8 T cells in the PG545-treated group compared with the PBS-treated group (23.2 ± 2.3 vs 12.9 ± 2.0; n = 3 per group; P < .05; Figure 5B). PG545 treatment also accelerated the rate of GVHD compared with PBS control injections (21 vs 29 days, P < .001; Figure 5C). These data support the hypothesis that HS can modulate the alloreactive T-cell response in vivo.
HS mimetic increases serum HS levels and increases CD8 T-cell proliferation in allo-HSCT recipients. BALB/c recipient of B10.D2 allo-BM + LC were treated with subcutaneous injections of the HS mimetic PG545 (20 mg/kg in PBS), or PBS control once weekly, beginning 1 day before allo-HSCT. (A) Serum HS levels were determined by ELISA on the indicated days after transplant; n = 2-4 samples each (*P < .05). (B) BrdU uptake by CD8 T cells 6 days after allo-HSCT. Average and SEM are plotted; n = 3 per group (*P < .05). (C) Survival analysis of allo-BM + LC+PG545 (n = 10) compared with allo-BM + LC + PBS (n = 10).
HS mimetic increases serum HS levels and increases CD8 T-cell proliferation in allo-HSCT recipients. BALB/c recipient of B10.D2 allo-BM + LC were treated with subcutaneous injections of the HS mimetic PG545 (20 mg/kg in PBS), or PBS control once weekly, beginning 1 day before allo-HSCT. (A) Serum HS levels were determined by ELISA on the indicated days after transplant; n = 2-4 samples each (*P < .05). (B) BrdU uptake by CD8 T cells 6 days after allo-HSCT. Average and SEM are plotted; n = 3 per group (*P < .05). (C) Survival analysis of allo-BM + LC+PG545 (n = 10) compared with allo-BM + LC + PBS (n = 10).
Recipient antigen-presenting cells are present at time of HS elevation
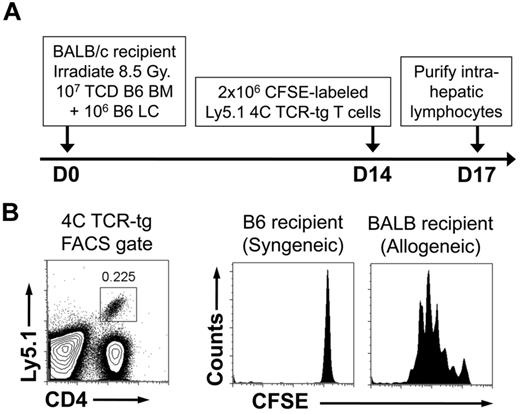
Because we hypothesize that HS stimulates recipient DCs to promote GVHD, we sought to demonstrate the persistence of recipient DCs at the time of HS elevation. For this purpose, we used the B6à BALB/c HSCT model of GVHD consisting of lethal irradiation and the transfer of 107 TCD-BM + 106 LCs. This model produces lethal GVHD within 30 days. At 14 days after transplant, we adoptively transferred 106 CFSE-labeled TCR-tg T cells from the 4C TCR-tg mouse. The 4C mouse is in the B6 background and has TCR-tg CD4+ T cells with direct allospecificity against the BALB/c MHC class II molecule I-Ad.22 TCR-tg T cells also were transferred into recipients of syngeneic HSCT on D14 as a control for homeostatic proliferation in potentially lymphopenic hosts (Figure 6A). As shown, in Figure 6B, there was robust proliferation of the TCR-tg T cells, demonstrating the presence of recipient APCs during the period of peak serum HS levels.
Persistence of recipient MHC class II–expressing cells after allogeneic HSCT. Lethally irradiated C57BL/6 recipients received 107 TCD-BM + 106 LC from either allogeneic BALB/c donors or syngeneic C57BL/6 donors. Fourteen days after transplant, recipient mice were injected with 2 × 106 CFSE-labeled lymphocytes from 4C TCR-tg mice (direct allospecificity toward the BALB/c MHC class II molecule I-Ad) that were on the Ly5.1 congenic background. Recipient intrahepatic lymphocytes were harvested 3 days later and FACS-analyzed. (A) Schematic of experiment. (B) FACS gates for detection of 4C TCR-tg T cells and CFSE analysis. Results shown are representative of 4 mice in each group.
Persistence of recipient MHC class II–expressing cells after allogeneic HSCT. Lethally irradiated C57BL/6 recipients received 107 TCD-BM + 106 LC from either allogeneic BALB/c donors or syngeneic C57BL/6 donors. Fourteen days after transplant, recipient mice were injected with 2 × 106 CFSE-labeled lymphocytes from 4C TCR-tg mice (direct allospecificity toward the BALB/c MHC class II molecule I-Ad) that were on the Ly5.1 congenic background. Recipient intrahepatic lymphocytes were harvested 3 days later and FACS-analyzed. (A) Schematic of experiment. (B) FACS gates for detection of 4C TCR-tg T cells and CFSE analysis. Results shown are representative of 4 mice in each group.
Serum HS elevation is associated with GVHD in patients undergoing allo-HSCT
We next determined whether the elevation in serum HS levels observed in the mouse model of GVHD correlated to those found in the clinical setting. Serum samples were obtained from human allo-HSCT recipients with and without clinical and/or pathologic evidence of acute GVHD (within 100 days of HSCT) as measured on a scale of grade 0 (no evidence of GVHD), grade 1 to 2 (mild GVHD), or grade 3 to 4 (severe GVHD). Patient demographics, indication for HSCT, donor MHC match, conditioning regimens, maintenance immunosuppression, and posttransplant infections were compared and found to be similar between groups (Table 1). Comparison of HS levels near the time of GVHD diagnosis demonstrated serum HS elevations that correlated with the severity of GVHD (grade 0, [HS] = 4.22 ± 0.39 μg/mL, n = 8; grade 1-2, [HS] = 10.89 ± 2.07 μg/mL, n = 17; grade 3-4, [HS] = 23.74 ± 4.66 μg/mL, n = 11; Figure 7A). Comparison of serum HS relative to the time of GVHD revealed a peak in serum HS levels that temporally correlated with the time of GVHD diagnosis (Figure 7B).
Characteristics of transplants according to degree of GVHD
| . | GVHD . | P* . | ||
|---|---|---|---|---|
| 0 (n = 8) . | 1-2 (n = 17) . | 3-4 (n = 11) . | ||
| Recipient factor | ||||
| Age, y | 48.0 ± 12.2 | 45.8 ± 13.3 | 44.5 ± 16.2 | .86 |
| Male, % | 87.5 | 58.8 | 72.7 | .38 |
| White, % | 100 | 76.5 | 72.7 | .31 |
| HSCT indication | ||||
| Acute myeloid leukemia | 4 | 5 | 2 | .83† |
| Acute lymphoblastic leukemia | 0 | 3 | 2 | |
| Chronic lymphocytic leukemia | 1 | 0 | 3 | |
| Hodgkin lymphoma | 0 | 3 | 0 | |
| Myelodysplastic syndrome | 1 | 0 | 2 | |
| Aplastic anemia | 1 | 2 | 0 | |
| Other | 1 | 4 | 2 | |
| Conditioning regimen | ||||
| Ablative | 3 | 4 | 4 | .79 |
| Nonablative/reduced intensity | 5 | 13 | 7 | |
| Total body irradiation | 1 | 1 | 2 | |
| Maintenance immunosuppression | ||||
| CellCept | 3 | 14 | 9 | .32 |
| Calcineurin inhibitor | 4 | 3 | 4 | |
| Prednisone | 1 | 3 | 2 | |
| Sirolimus | 0 | 0 | 1 | |
| None | 1 | 0 | 0 | |
| Donor-recipient MHC match | ||||
| 6/6 | 7 | 10 | 5 | .19‡ |
| 2-5/6 | 1 | 6 | 6 | |
| 0/6 | 0 | 1 | 0 | |
| Infection within 100 days of transplant | ||||
| CMV viremia | 2 | 10 | 7 | .50 |
| Bacteremia | 0 | 0 | 1 | |
| . | GVHD . | P* . | ||
|---|---|---|---|---|
| 0 (n = 8) . | 1-2 (n = 17) . | 3-4 (n = 11) . | ||
| Recipient factor | ||||
| Age, y | 48.0 ± 12.2 | 45.8 ± 13.3 | 44.5 ± 16.2 | .86 |
| Male, % | 87.5 | 58.8 | 72.7 | .38 |
| White, % | 100 | 76.5 | 72.7 | .31 |
| HSCT indication | ||||
| Acute myeloid leukemia | 4 | 5 | 2 | .83† |
| Acute lymphoblastic leukemia | 0 | 3 | 2 | |
| Chronic lymphocytic leukemia | 1 | 0 | 3 | |
| Hodgkin lymphoma | 0 | 3 | 0 | |
| Myelodysplastic syndrome | 1 | 0 | 2 | |
| Aplastic anemia | 1 | 2 | 0 | |
| Other | 1 | 4 | 2 | |
| Conditioning regimen | ||||
| Ablative | 3 | 4 | 4 | .79 |
| Nonablative/reduced intensity | 5 | 13 | 7 | |
| Total body irradiation | 1 | 1 | 2 | |
| Maintenance immunosuppression | ||||
| CellCept | 3 | 14 | 9 | .32 |
| Calcineurin inhibitor | 4 | 3 | 4 | |
| Prednisone | 1 | 3 | 2 | |
| Sirolimus | 0 | 0 | 1 | |
| None | 1 | 0 | 0 | |
| Donor-recipient MHC match | ||||
| 6/6 | 7 | 10 | 5 | .19‡ |
| 2-5/6 | 1 | 6 | 6 | |
| 0/6 | 0 | 1 | 0 | |
| Infection within 100 days of transplant | ||||
| CMV viremia | 2 | 10 | 7 | .50 |
| Bacteremia | 0 | 0 | 1 | |
Kruskal-Wallis for continuous variables; Fisher exact test for categorical variables.
Acute leukemia vs others.
MHC match: 6/6 vs 0-5/6.
HS is elevated in serum samples of human allo-HSCT recipients with GVHD. (A) Serum samples from allo-HSCT recipients were tested for HS by ELISA. Patients were divided into 3 groups: no GVHD (grade 0; n = 8), mild GVHD (grade 1-2; n = 17), and moderate to severe GVHD (grade 3-4; n = 11; *P = .003, **P = .0009, ***P = .01). (B) Serum HS levels relative to time of diagnosis of GVHD in patients with grades 1 to 2 and grade 3 to 4 GVHD. Average ± SEM plotted (*P = .01).
HS is elevated in serum samples of human allo-HSCT recipients with GVHD. (A) Serum samples from allo-HSCT recipients were tested for HS by ELISA. Patients were divided into 3 groups: no GVHD (grade 0; n = 8), mild GVHD (grade 1-2; n = 17), and moderate to severe GVHD (grade 3-4; n = 11; *P = .003, **P = .0009, ***P = .01). (B) Serum HS levels relative to time of diagnosis of GVHD in patients with grades 1 to 2 and grade 3 to 4 GVHD. Average ± SEM plotted (*P = .01).
Discussion
Recent studies have revealed an important role for exogenous TLR ligands in promoting acute rejection in the setting of solid organ transplantation,14-16 as well as in accelerating the severity of GVHD after allo-HSCT.17 Here, we provided evidence for the first time that HS, an endogenous TLR4 ligand, was released during the onset of GVHD and may have a role in promoting GVHD. This is particularly relevant because the onset of GVHD usually occurs in the absence of obvious exogenous TLR stimuli. More importantly, the observations that inhibition of HS release by A1AT resulted in a significant improvement in GVHD and survival in mice and that serum HS levels were correlated to the severity of GVHD in humans suggest that blockade of HS release after allo-HSCT may have therapeutic potential for the control of clinical GVHD.
The role of TLR activation on APCs in promoting adaptive immune responses has been well described.29 Recent studies have shown that TLRs also are expressed on T cells30,31 and that direct TLR2 and TLR9 signaling in T cells is critical for T-cell activation and survival in response to viral infections.32,33 In the setting of alloreactive T-cell responses, we found no significant difference in proliferation of MyD88−/− T cells stimulated with HS compared with the WT T-cell controls. Instead, the HS-dependent enhancement of alloreactivity in vitro was mediated by activating the TLR4-MyD88 and TRIF pathway in APCs. This is in line with the previous observation that the exogenous TLR ligand CpG promotes allogenic T-cell activation and GVHD via activating the TLR9 pathway in APCs,17 but contrasts to findings by Li et al using MyD88- and TRIF-deficient HSCT recipients.34 The reasons for the differential roles of direct TLR signaling in T-cell activation in different settings remain unknown but could be influenced by other T-cell activation factors such as the strength of TCR stimulation, the intensity of costimulation, as well as different cytokine milieus that occur in different strain models of GVHD.
Previous studies have shown that recipient APC of hematopoietic lineage are rapidly depleted after allo-HSCT.35-38 Thus, we determined whether any recipient APC was present at the time of HS elevation in GVHD. Using an adoptive transfer model in which alloreactive donor-strain TCR-tg T cells with direct alloreactivity against recipient MHC class II are transferred to allo-HSCT recipients, we found that donor APCs are present when previous studies have shown near complete depletion of recipient hematopoietic APCs. Although this experiment provides evidence that recipient MHC class II–expressing cells are present at the time of HS elevation, it remains unclear as to which type of cells are expressing the MHC class II. Either the in vivo functional assay using TCR-tg T cells is more sensitive at finding rare recipient DCs or macrophages present at the time of GVHD, or that the MHC class II is expressed on nonhematopoietic cell lineages, such as endothelial cells that can serve as effective APCs.39 Alternatively, HS may activate donor APCs that are capable of presenting recipient alloantigen to donor T cells through indirect antigen presentation.
It has been suggested that myeloablative conditioning regimens such as chemotherapy and irradiation can cause injury to the bowel, which can release DAMPs and allow PAMP-producing bacterial to translocation across the bowel epithelium.40 However, clinical GVHD often occurs weeks or months after transplantation and the contribution of tissue damage from conditioning regimens is not clearly linkable to these episodes. In this study, we found that HS did not become elevated in the serum as a consequence of irradiation, bone marrow transplantation, or from the reconstitution of syngeneic lymphocyte populations. Instead, it became highly elevated at the onset of clinical GVHD in the serum of recipients that received allogeneic lymphocytes, suggesting HS release is related to the alloreactive T-cell response involved in GVHD. We also observed that serum HS levels were elevated near the time of GVHD diagnosis in patients undergoing allo-HSCT and correlated with the severity of GVHD. However, given the heterogeneity of the patients involved in this study and the small sample size, further confirmation would be needed in future studies.
How does the activation of alloreactive T cells lead to the release of HS and the subsequent augmentation of GVHD? Previous studies have shown that the extracellular matrix molecule syndecan-1 is detectable in the serum of HSCT recipients with acute GVHD and that its serum concentration correlates with GVHD severity.41 These results indicate that the extracellular matrix undergoes degradation during GVHD. It also has been shown that activated T cells can produce an extracellular matrix-degrading endoglycosidase capable of degrading the proteoglycan scaffold of the extracellular matrix to generate soluble HS.42 Furthermore, a recent report has shown that heparanase treatment of mice is protective against GVHD in the setting of allo-HSCT.43 Heparanase therapy also has been shown to decrease the severity of disease in mouse models of experimental autoimmune encephalitis44 and type I diabetes mellitus,45 both T cell–mediated diseases. Taken together, these observations suggest a possible mechanism whereby tissue invading alloreactive T cells degrade the extracellular matrix and generate soluble HS at the onset of GVHD. The increased HS levels could then serve as a positive feedback mechanism that augments the allogeneic T-cell response and further advances GVHD.
A1AT has been shown to suppress autoimmune diabetes caused by invasive insulitis in mice,46 to induce immune tolerance after islet allograft transplantation,27 and recently to reduce alloreactivity and GVHD in experimental models of allo-HSCT.47,48 Although inhibition of IL-32 activation,47 reduced production of proinflammatory cytokines, and enhanced secretion of the anti-inflammatory cytokine IL-1048 have been implicated in the action of A1AT, the underlying mechanisms responsible for the suppressive effect of A1AT in alloreactivity and GVHD remain incompletely defined. Our findings that HS is a potent stimulator of alloreactive T cells and that administration of A1AT decreased serum HS levels, leading to decreased alloreactive T-cell responses, provide additional mechanistic insight into the action of A1AT in the suppression of alloreactive T-cell responses and GVHD.
In conclusion, we have shown that HS can promote alloreactive T-cell responses in vitro. In mice, serum HS levels are acutely elevated at the onset of clinical GVHD after allo-HSCT. Treatment with A1AT decreases HS levels, leading to a reduction in alloreactive T-cell responses and an improvement in GVHD. Conversely, an HS mimetic that increases serum HS levels accelerates GVHD. In patients undergoing allo-HSCT, serum HS level elevations correlate with the severity of GVHD. These results identify a new role for HS in promoting acute GVHD after allo-HSCT, and they suggest that modulation of HS release may have implications in the control of clinical GVHD.
There is an Inside Blood commentary on this article in this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants CA136934 (Y.Y.) and CA047741 (N.J.C.) from the National Institutes of Health; by a grant from the Alliance for Cancer Gene Therapy (Y.Y.); and by grants from the American Association for the Study of Liver Disease (T.V.B.) and the American Society of Transplantation (T.V.B.).
National Institutes of Health
Authorship
Contribution: T.V.B. designed and performed research, collected and analyzed data, and wrote the paper; L.L. performed research and collected and analyzed data; D.M.C., Z.L., and K.D. analyzed data; and X.H., N.J.C., and Y.Y. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: K.D. is an employee of Progen Pharmaceuticals Limited, Queensland, Australia. The remaining authors declare no competing financial interests.
Correspondence: Yiping Yang, Departments of Medicine and Immunology, Duke University Medical Center, Box 103005, Durham, NC 27710; e-mail: yang0029@mc.duke.edu.

![Figure 1. HS is a potent stimulator of alloreactive T-cell responses through the TLR4- and MyD88-dependent activation of DCs. (A) TLR and NLR agonists were assayed in allogeneic T-cell proliferation assay between purified T cells (2 × 105/well) from C57BL/6 mice and bone marrow–derived BALB/c DCs (2.5 × 104/well). Cells were cocultured either alone (media) or in the presence of LPS (100 ng/mL), Pam3CSK4 (2 μg/mL), hyaluronan (HA; 100 μg/mL), sonicated-HA (sHA; 100 μg/mL), fibronectin (FN; 100 μg/mL), fibrinogen (Fbn; 100 μg/mL), HS (100 μg/mL), Hsp70 (5 μg/mL), HMGB1 (1 μg/mL), C12-iE-DAP (1 μg/mL), or L18-MDP (1 μg/mL) for 72 hours and then pulsed [3H]thymidine for 16 hours. Proliferation was determined by 3H incorporation and results are expressed as cpm ± SEM. Baseline alloreactivity is indicated by the dotted line (*P < .05 compared with media alone). (B) Proliferation performed as described in panel A ± the addition of the LPS inhibitor polymyxin B (PMB; 10 μg/mL; *P < .05). (C) Proliferation assay performed as described in panel A with purified responder T cells (R) from either WT (+) or MyD88−/− (−) C57BL/6 mice were cocultured with DC stimulators (S) from either WT (+) or MyD88−/− (−) BALB/c mice (*P < .05 compared with media alone in S+/R+ group. (D-E) Analysis of proliferation and IFN-γ production in proliferation assays performed as described in panel A using WT, TLR4−/−, and MyD88−/− BALB/c DCs as stimulators and purified C57BL/6 T cells as responders (*P < .05). Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/14/10.1182_blood-2011-07-368720/4/m_zh89991295140001.jpeg?Expires=1765941474&Signature=loDWji1FnVaVVorkTlf5fm2HOLNh4ldWo~P4OVC4Al1Td7qgf5i~d0QPrntkXKm5EsafFZb0L44ymaxFu6Z2ptEyWJnKIPinvYUokmflbXqVPpfIPWBDo~T-W49u~BLotxpBRFBODXZtuFZWXYS1b3YCnJyg27zst6rmiQtkOjRxBflTfiADJNjFwLQdSYFCBsETq36BGkXGrz8Xs~8svcIKhXsAN3yTaNXYjlZ~wSWpDrQgV35f9dw6CHJ6eMNS9TWHk5tEL1oeLOgHY4aZsNVJJQIGFt~Rr39f4haEAhzQZ54KUf~L0OzJ0G-X7F~jt5JM~7DYsD3pBU3WOEi8Zg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal