Abstract
Prothrombin activation can proceed through the intermediates meizothrombin or prethrombin-2. To assess the contributions that these 2 intermediates make to prothrombin activation in tissue factor (Tf)–activated blood, immunoassays were developed that measure the meizothrombin antithrombin (mTAT) and α-thrombin antithrombin (αTAT) complexes. We determined that Tf-activated blood produced both αTAT and mTAT. The presence of mTAT suggested that nonplatelet surfaces were contributing to approximately 35% of prothrombin activation. Corn trypsin inhibitor–treated blood was fractionated to yield red blood cells (RBCs), platelet-rich plasma (PRP), platelet-poor plasma (PPP), and buffy coat. Compared with blood, PRP reconstituted with PPP to a physiologic platelet concentration showed a 2-fold prolongation in the initiation phase and a marked decrease in the rate and extent of αTAT formation. Only the addition of RBCs to PRP was capable of normalizing αTAT generation. FACS on glycophorin A–positive cells showed that approximately 0.6% of the RBC population expresses phosphatidylserine and binds prothrombinase (FITC Xa·factor Va). These data indicate that RBCs participate in thrombin generation in Tf-activated blood, producing a membrane that supports prothrombin activation through the meizothrombin pathway.
Introduction
Prothrombin activation by prothrombinase (IIase or factor Xa [fXa]·fVa) involves cleavage at Arg271 and Arg320 to produce the α-thrombin (αIIa) product.1,2 Depending on the order of cleavage, activation occurs via 2 possible intermediates; meizothrombin (mIIa) or prethrombin-2. mIIa arises from initial cleavage at Arg320, yielding a membrane-binding enzyme; initial cleavage at Arg271 yields an inactive noncovalent complex of fragment 1.2 and prethrombin-2. Cleavage at the alternate Arg residue yields αIIa.3 On synthetic phospholipid vesicles, prothrombin activation by IIase proceeds exclusively through the mIIa pathway,3 with the probable mechanism involving mIIa dissociating from the IIase complex and then rebinding to IIase to yield αIIa.4,5 On washed platelets, prothrombin activation proceeds through the prethrombin-2 pathway,6 with no detectable mIIa being released from the platelet surface. We confirmed these previous results with washed platelets under static and flow conditions.7 However, mIIa has been observed in clotting blood,8 suggesting that prothrombin activation in blood involves both cleavage pathways. The relative prevalence of these 2 pathways in blood has potentially interesting regulatory consequences for hemostasis. Although the anticoagulant potential of mIIa as a protein C activator when in complex with thrombomodulin is similar to that of αIIa,5 mIIa displays poor activity toward the procoagulant substrates fibrinogen, platelets, and fV; displays a reduced vulnerability to antithrombin (AT); and is a more potent vasoconstrictor.9
Platelets are thought to provide the primary surface for prothrombin activation. The observation that the IIase activation pathway on platelets does not release mIIa raises 2 important questions: (1) what is the source of mIIa reported in clotting blood7 and (2) how significant are nonplatelet cell surfaces to overall thrombin generation?
We used a commercial ELISA assay (Enzygnost TAT Micro; Siemens Healthcare) that measures both mIIa AT (mTAT) and α-thrombin AT (αTAT) to study thrombin generation in tissue factor (Tf)–activated whole blood (WB).10,11 For the present study, we devised immunoassays capable of selective quantitation of mTAT and αTAT species and used these assays to examine the contributions of various cell surfaces to thrombin generation.
Methods
All materials used in the present study were of reagent grade and quality. For a complete list, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
WB fractionation
Volunteers signed informed consent documents in accordance with the Declaration of Helsinki and our methods were approved by the University of Vermont Committee on Human Subjects. WB was drawn via phlebotomy (the first 3 mL was discarded) and mixed with 0.1 mg/mL of corn trypsin inhibitor (CTI). Three milliliters of WB was also drawn into 3.2% trisodium citrate (9:1) and a complete blood count performed on an automated hematology analyzer (pocH-100i; Sysmex). Aliquots of CTI WB were left at room temperature while the remaining CTI WB was centrifuged at 150g or 1100g for 10 minutes. After centrifugation, the top layers of platelet-rich plasma (PRP)/buffy coat (150g) or platelet-poor plasma (PPP)/packed RBCs (1100g) were removed from their respective preparations. These steps yielded 5 fractions: WB, PRP, buffy coat, PPP, and RBCs. PRP was reconstituted to each subject's physiologic platelet concentration with the aforementioned blood fractions to enrich each cell type.
WB activation experiments
The procedure used for the WB activation experiments was a modification of that described by Brummel et al.11 CTI blood or blood fractions were aliquoted into rocking tubes at 37°C containing 5pM relipidated Tf1-243. Tubes were quenched 1:1 with 50mM EDTA, 20mM benzamidine, and 100μM Phe-Pro-Arg chloromethylketone at defined intervals for 20 minutes. Samples were centrifuged at 1200g for 30 minutes at 4°C, and both the soluble and sedimented materials were frozen at −80°C.
αTAT and mTAT ELISAs
The αTAT and mTAT assays are sandwich-style ELISAs. Plates were coated with 5 μg/mL of capture Ab (anti–human αIIa or anti–human prothrombin fragment 2 for αTAT and mTAT, respectively) for 16 hours before use and stored at 4°C. Plates were washed and blood serum samples were diluted before incubation for 2 hours at room temperature. Plates were washed and probed with biotinylated burro anti–human AT at 5 μg/mL in HBS and 1% BSA, and incubated at room temperature for 1 hour. Plates were then washed and binding was detected using HRP-streptavidin and a chromogenic substrate on a microplate reader (EL 312e; BioTek). A complete description of the assay procedure, sample, and standard preparation can be found in the supplemental materials.
Washed RBCs
Blood was drawn via phlebotomy into 0.1 mg/mL of CTI and 10-mL aliquots were centrifuged in 15-mL tubes at 150g for 10 minutes. After centrifugation, the top layer of PRP/buffy coat plus the underlying 1 mL of RBCs were aspirated and discarded. Next, 6 mL of RBC wash buffer (21mM Tris-base, 140mM NaCl, 11.1mM dextrose, 4.7mM KCl, 2mM CaCl2, 1.2mM MgSO4, and 0.1% PEG 8000 at pH 7.4) were added and the tubes mixed via inversion. This wash step was repeated twice, followed by 3 wash cycles at 1100g. The cells were stored at 25°C and RBC purity was measured via a complete blood count in an automated hematology analyzer (pocH-100i; Sysmex).
PMA treatment of RBCs
Washed RBCs were diluted to 2% in wash buffer and mixed with an equal volume of wash buffer containing 4, 8, 12, or 16μM phorbol 12-myristate 13-acetate (PMA; 2, 4, 6, 8μM final concentrations) and incubated at room temperature for 30 minutes. The PMA-treated cells were then centrifuged at 1100g for 5 minutes before being washed 3 times. Treated cells were diluted to 2 × 106 cell/mL in RBC wash buffer and phosphatidylserine (PS) exposure was detected with FITC-bovine lactadherin or FITC-EGRck-fXa (15nM) in the presence of saturating FVa (20nM) on a flow cytometer (FC500; Beckman Coulter).
PS expression on RBCs
Samples for FACS were prepared as described previously.12 For positive controls, washed RBCs were diluted to 20% hematocrit in RBC wash buffer and incubated with 10mM N-ethylmaleimide (NEM) at 37°C for 30 minutes. NEM-treated cells were then washed 3 times before incubation with a 4μM concentration of the calcium ionophore A23187 at 37°C for 30 minutes. Ionophore-treated cells were washed 3 times and diluted to 2 × 106 cell/mL in RBC wash buffer. Samples were incubated and washed in the same manner as the controls but without NEM or ionophore treatment. Reticulocytes were detected with FITC–anti-cd71. Platelet and WBC contaminants were detected with PE–anti-cd42b and PE–anti-cd45, respectively. RBCs were detected using PE–anti-glycophorin A (cd235a). PS expression was evaluated via the binding of FITC-labeled bovine lactadherin, FITC-labeled annexin V, or FITC-EGRck–labeled fXa (15nM) in the presence of saturating fVa (20nM) on a flow cytometer (FC500; Beckman Coulter).
Prothrombinase
Washed RBCs were prepared as described in “Washed RBCs.” IIase experiments were constructed from 3 stock solutions. Solution A contained washed RBCs that were concentrated to 90% hematocrit in wash buffer. Solution B contained 40nM fVa and 400pM fXa in RBC wash buffer. Solution C contained 2.8μM prothrombin and 6.8μM AT. For the typical reaction, 480 μL of solution A was premixed with 360 μL of solution B for 1 minute at 37°C to allow for the assembly of IIase. The reaction was initiated by the addition of 360 μL of solution C (final concentrations, 1.4μM fII, 3.4μM AT, 20nM fV, and 200pM fXa). For controls, solution A was replaced by 50 μL of RBC wash buffer supplemented with 250nM phosphatidylcholine-phosphatidylserine (PCPS) or 50 μL of RBC wash supernatant that was prepared via centrifugation of solution A at 1100g for 5 minutes. This concentration of PCPS resulted in a level of αTAT generation equivalent to that of a physiologic concentration (200 × 103/μL) of washed platelets. Samples were quenched 1:1 with 50mM EDTA, 20mM benzamidine, and 100μM Phe-Pro-Arg chloromethylketone at defined intervals for 20 minutes. All samples were centrifuged at 1100g for 5 minutes at 4°C and the soluble material frozen at −80°C. Western blotting of nonreduced prothrombinase supernatants was performed as described previously.10
Computational model
Computational Tf-initiated thrombin-generation profiles were produced as described previously.13 Concentrations of zymogens (fII, fVII, fIX, and fX), cofactors (fV and fVIII), and inhibitors (AT and Tf pathway inhibitor) were set to mean physiologic concentrations. Prothrombin activation in this model mechanistically occurs exclusively through the mIIa pathway with release of the mIIa intermediate from IIase. mIIa either reacts with AT to form mTAT or with IIase to yield αIIa and ultimately αTAT. The model was modified to reflect empirical measurements5 showing that the second-order rate constant describing mIIa inhibition by AT is approximately 25% of that for αIIa.
Results
Quantitation of αTAT and mTAT
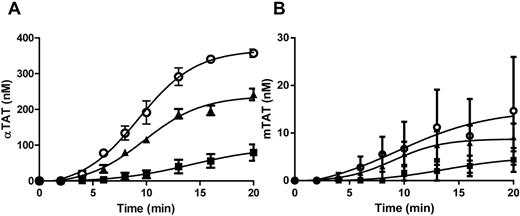
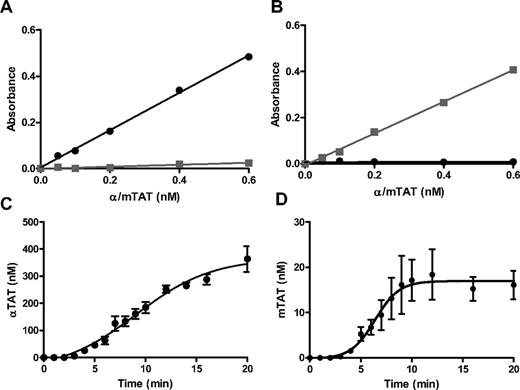
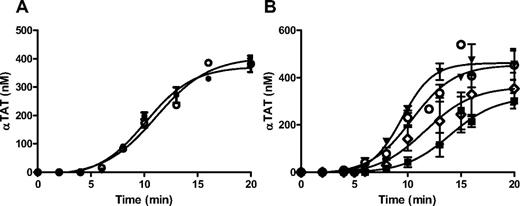
The thrombin species produced in Tf-activated WB were quantitated using immunoassays using mAbs to αIIa (αTAT; βTAT and γTAT are also recognized) or prothrombin fragment 2 (mTAT) to capture their respective antigens. Standards were prepared by reacting human αIIa or ecarin-activated recombinant human prothrombin (R155A, R271A, and R284A) with a 2-fold molar excess of human AT. As shown in Figure 1A (αTAT) and 1B (mTAT), each assay recognizes its respective antigen over a concentration range of 50-600pM with an intra/interassay coefficients of variation of 2.6/3.8 and 5.1/8.3, respectively.
αTAT and mTAT ELISAs. (A) Human αTAT (●) and mTAT (■) captured with an anti–human thrombin mAb and probed with a biotin-labeled anti–human AT polyclonal Ab. (B) Human αTAT (●) and mTAT (■) captured with an anti–human prothrombin fragment 2 mAb and probed with a biotin-labeled anti–human AT polyclonal Ab. (C) αTAT ELISA analyses of WB blood time courses from 4 subjects at 5pM (●) relipidated rTf. Data are shown as the means ± SEM (n = 4). (D) mTAT ELISA analyses of WB blood time courses from 4 subjects at 5pM (●) relipidated rTf. Data are shown as the means ± SEM (n = 4).
αTAT and mTAT ELISAs. (A) Human αTAT (●) and mTAT (■) captured with an anti–human thrombin mAb and probed with a biotin-labeled anti–human AT polyclonal Ab. (B) Human αTAT (●) and mTAT (■) captured with an anti–human prothrombin fragment 2 mAb and probed with a biotin-labeled anti–human AT polyclonal Ab. (C) αTAT ELISA analyses of WB blood time courses from 4 subjects at 5pM (●) relipidated rTf. Data are shown as the means ± SEM (n = 4). (D) mTAT ELISA analyses of WB blood time courses from 4 subjects at 5pM (●) relipidated rTf. Data are shown as the means ± SEM (n = 4).
mTAT and αTAT levels in Tf-activated blood showed that αTAT levels were much greater than mTAT levels. Given this concentration difference, the contribution of αTAT present in a sample to the signal measured in that same sample using the mTAT assay was converted. A series of concentrations of αTAT (50-500nM) were added to citrate plasma and then assayed in the mTAT ELISA (data not shown). Graphical analyses relating the αTAT concentration in the sample to the apparent mTAT concentration showed that the contribution by αTAT to the mTAT signal was equivalent in magnitude to 1% of the αTAT concentration in the sample. All reported mTAT data are therefore corrected for the contributions of αTAT. Given the lesser concentrations of mTAT and αTAT in the samples, no corrections to the αTAT data were made.
The αTAT produced in the serum of Tf-activated blood from 4 healthy subjects over 20 minutes (Figure 1C) was analyzed. The data show αTAT generation of 27.2 ± 3.7nM/min (mean ± SEM) after a 3-minute lag phase, reaching an average maximum αTAT level of 364 ± 55nM. mTAT generation was concurrent with that of αTAT; however, both the rate (2.6 ± 0.9nM/min) and maximum level (16.1 ± 3.1nM) were considerably decreased (Figure 1D).
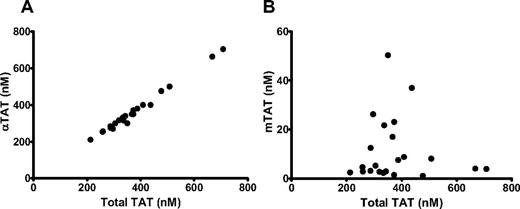
The αTAT and mTAT species were examined in relation to the total TAT at the 20-minute time point in the WB of 10 healthy subjects over 22 blood draws (Figure 2). As shown previously,14 the peak levels of αTAT varied widely from 383 ± 65nM (mean ± SEM) between subjects, displaying a “normal” range between 200 and 700nM. mTAT levels also varied (11.3 ± 2.8nM, range of 1-50nM), with the proportion of mTAT to αTAT variable both within and between subjects. For example, across 4 blood draws in 1 subject, we measured 2, 8, 26, and 50nM mTAT and αTAT concentrations of 331, 380, 270, and 300nM, respectively.
αTAT and mTAT levels versus total TAT levels in individual patients. WB was drawn via phlebotomy into 0.1 mg/mL of CTI and subjected to 5pM relipidated rTf. Samples were quenched at 20 minutes with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA (A) or mTAT ELISA (B). n = 10, and blood from some subjects was drawn more than once.
αTAT and mTAT levels versus total TAT levels in individual patients. WB was drawn via phlebotomy into 0.1 mg/mL of CTI and subjected to 5pM relipidated rTf. Samples were quenched at 20 minutes with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA (A) or mTAT ELISA (B). n = 10, and blood from some subjects was drawn more than once.
To investigate the variability of mTAT levels, αTAT and mTAT were added to WB and measured after processing. When αTAT is spiked into WB and the blood activated with Tf, αTAT supplementation is additive with respect to the levels generated in the control (no exogenous TAT). αTAT added to prequenched blood or its derived plasma shows no losses after processing when the soluble fraction is assayed. In contrast, when mTAT is spiked into WB and the blood subsequently activated with TF, only 40% of the antigen is recovered in the soluble fraction. When mTAT is spiked into prequenched WB, only 70% is recovered, whereas mTAT spiked into plasma derived from prequenched blood is 100% recovered. These data suggest that there are 2 sources of variation in the levels of mTAT measured in the soluble phase of our quenched time course samples: adsorption to cells and proteolytic degradation.
Computational analysis of empirical mTAT generation
mIIa production by prothrombinase in purified systems has been studied extensively on synthetic phospholipid vesicles.3,15,16 In the absence of AT, prothrombin is rapidly activated through the mIIa pathway, with maximum mIIa levels transiently reaching 40% of the final αIIa concentration. In Tf-activated WB, the generation of mIIa results in partitioning of the mIIa between the reaction with the stoichiometric inhibitor AT and rebinding to prothrombinase and conversion to αIIa, with subsequent inhibition resulting in αTAT. Figure 3A shows the results of computational analysis quantifying the extent of this partitioning. Predicted time courses for αTAT and mTAT generation after a hypothetical 5pM Tf stimulus are shown. The computational analysis predicted that, although all of the prothrombin was converted through mIIa, a maximum of 11% of the mIIa was trapped as mTAT rather than being converted to αIIa and eventually trapped as αTAT. Differences in proteome composition also affect the relative distribution of mTAT and αTAT species.17
Computational simulation of the meizothrombin pathway. All coagulation factors and inhibitors are set to their mean physiologic levels. Thrombin generation was initiated with a hypothetical 5pM Tf stimulus. (A) αTAT (■) and mTAT (■) species. (B) Empirical αTAT data from Figure 1C (●) and Figure 1D were used to derive αTAT species originating through the mIIa pathway (○).
Computational simulation of the meizothrombin pathway. All coagulation factors and inhibitors are set to their mean physiologic levels. Thrombin generation was initiated with a hypothetical 5pM Tf stimulus. (A) αTAT (■) and mTAT (■) species. (B) Empirical αTAT data from Figure 1C (●) and Figure 1D were used to derive αTAT species originating through the mIIa pathway (○).
Given the evidence that platelets support the prethrombin-2 pathway,6 the detection of mTAT in Tf-activated blood suggests that a nonplatelet surface contributes to prothrombin activation through the mIIa intermediate. Based on the model's predicted distribution of αTAT and mTAT, we can estimate (Figure 3B) that the concentration of mTAT at any time point represents approximately 11% of the mIIa produced to that point, with the remainder further processed to αIIa and αTAT. Overall, the maximum level of mTAT (16.1nM) in these 4 subjects suggests that approximately 144nM prothrombin was processed through the mIIa pathway; of this, 128nM mIIa would have been further processed to αIIa and ultimately trapped as αTAT. Therefore, approximately 35% of the αTAT measured at 20 minutes (364nM) would have been derived from the mIIa pathway.
Cellular contributions to thrombin generation
To examine the potential for blood fractions other than platelets to generate αIIa, blood was fractionated to produce packed RBCs, PRP, PPP, and buffy coat. Various combinations of these fractions (PRP + PPP, PRP + WBCs, and PRP + RBCs) were constructed and their response to a 5pM Tf stimulus was compared with that of the source WB. In all cases, reconstitution of PRP with other fractions was performed to achieve the physiologic platelet and peripheral blood cell concentrations measured in each subject's intact blood. To do so, cell counts were performed on each of the blood fractions and reconstituted mixtures. Table 1 presents these results. The cell counts for each blood mixture are displayed as a ratio of the cell counts in the reconstituted fraction over the subject's WB.
Cell counts measured in the reconstituted PRP fractions
| . | Reconstituted fraction/WB ratio . | |||
|---|---|---|---|---|
| Platelets . | WBCs . | RBCs . | Clot time . | |
| WB | 1 | 1 | 1 | 1 |
| PRP + PPP | 1.08 ± 0.11 | 0.08 ± 0.06 | < 0.002 | 1.89 ± 0.2 |
| PRP + buffy coat | 1.22 ± 0.06 | 1.05 ± 0.16 | 0.07 ± 0.02 | 1.83 ± 0.26 |
| PRP + RBCs | 1.20 ± 0.06 | 0.19 ± 0.04 | 0.68 ± 0.13 | 1.14 ± 0.08 |
| . | Reconstituted fraction/WB ratio . | |||
|---|---|---|---|---|
| Platelets . | WBCs . | RBCs . | Clot time . | |
| WB | 1 | 1 | 1 | 1 |
| PRP + PPP | 1.08 ± 0.11 | 0.08 ± 0.06 | < 0.002 | 1.89 ± 0.2 |
| PRP + buffy coat | 1.22 ± 0.06 | 1.05 ± 0.16 | 0.07 ± 0.02 | 1.83 ± 0.26 |
| PRP + RBCs | 1.20 ± 0.06 | 0.19 ± 0.04 | 0.68 ± 0.13 | 1.14 ± 0.08 |
Complete blood counts were performed on citrated samples from each blood mixture on an automated hematology analyzer. Cell counts were adjusted for the addition of 3.2% sodium citrate (9:1). Data are presented as the ratio of the reconstituted fraction over each patient's WB. Data are presented as the means ± SEM (n = 5).
CTI blood, “mock-fractionated” via centrifugation at 150g followed by remixing (Figure 4A) before activation by Tf, showed no difference in αTAT generation compared with unprocessed WB. αTAT-generation profiles for the reconstituted mixtures are shown in Figure 4B and the thrombin-generation parameters are summarized in Table 2. WB from the 5 subjects in the cohort clotted in 4:09 ± 0:19 minutes, generated αTAT at a maximum rate of 47 ± 7nM/min, and reached a maximum αTAT level of 444 ± 50nM. PRP reconstituted to each subject's physiologic platelet concentration with PPP showed a marked delay in the onset of thrombin generation, clotting in 7:54 ± 1:12 minutes, generated αTAT at a rate of 38 ± 5nM/min, and reached a maximum level of 267 ± 20nM (60% of WB). The addition of buffy coat to PRP was incapable of normalizing the clot time (7:46 ± 1:35 minutes) and had a modest (approximately 15%) negative effect on the maximum rate (32 ± 6nM/min) and a positive effect (approximately 33%) on the maximum level (335 ± 54nM) of αTAT generation (75% of WB). The addition of packed RBCs to PRP normalized the IIa generation parameters. PRP reconstituted with a physiologic level of RBCs showed little change in lag phase compared with the corresponding WB (4:47 ± 0:25 minutes), generated αTAT at the same rate (48 ± 7nM/min), and reached the same maximum level of αTAT (451 ± 51nM). When fXIa was used as the initiator of the reaction, the differences in thrombin generation between the various fractions were unchanged, suggesting that these phenomena are independent of the initiator. These data suggest that RBCs are a significant contributor to thrombin generation.
αTAT ELISA analyses of reconstituted PRP. WB was drawn via phlebotomy into 0.1 mg/mL of CTI and subjected to differential centrifugation to obtain WB, PRP, PPP, buffy coat, and packed RBCs. An aliquot of CTI blood was “mock fractionated” via centrifugation at 150g, followed by remixing before activation by Tf. (A) αTAT ELISA analyses were performed on a time course from a representative subject's WB (○) and mock fractionated blood (●) that was subjected to 5pM relipidated rTf. Samples were quenched at various intervals with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA. (B) CTI blood was subjected to differential centrifugation to obtain WB, PRP, PPP, buffy coat (WBC surrogate), and packed RBCs. The latter 3 fractions were used to reconstitute PRP to each subject's physiologic platelet concentration. αTAT ELISA analyses were performed on blood fraction time courses from 5 subjects WB (○), PRP + PPP (■), PRP + buffy coat (♢), and PRP + packed RBCs (▾) subjected to 5pM relipidated rTf. Samples were quenched at various intervals with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA. Data are shown as the means ± SEM (n = 5).
αTAT ELISA analyses of reconstituted PRP. WB was drawn via phlebotomy into 0.1 mg/mL of CTI and subjected to differential centrifugation to obtain WB, PRP, PPP, buffy coat, and packed RBCs. An aliquot of CTI blood was “mock fractionated” via centrifugation at 150g, followed by remixing before activation by Tf. (A) αTAT ELISA analyses were performed on a time course from a representative subject's WB (○) and mock fractionated blood (●) that was subjected to 5pM relipidated rTf. Samples were quenched at various intervals with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA. (B) CTI blood was subjected to differential centrifugation to obtain WB, PRP, PPP, buffy coat (WBC surrogate), and packed RBCs. The latter 3 fractions were used to reconstitute PRP to each subject's physiologic platelet concentration. αTAT ELISA analyses were performed on blood fraction time courses from 5 subjects WB (○), PRP + PPP (■), PRP + buffy coat (♢), and PRP + packed RBCs (▾) subjected to 5pM relipidated rTf. Samples were quenched at various intervals with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA. Data are shown as the means ± SEM (n = 5).
Thrombin-generation parameters from analyses of reconstituted PRP time courses
| Mixture . | Clot time, min . | αTAT max rate, nM/min . | αTAT max level, nM . | αTAT max % of WB . |
|---|---|---|---|---|
| WB | 4:09 ± 0:19 | 47.2 ± 7.2 | 444 ± 49.6 | 100 |
| PRP + PPP | 7:54 ± 1:12 | 38 ± 4.9 | 267 ± 19.7 | 60 |
| PRP + buffy coat | 7:46 ± 1:35 | 32.4 ± 6.3 | 335 ± 53.7 | 75 |
| PRP + RBCs | 4:47 ± 0:25 | 48.4 ± 7.0 | 451 ± 51.4 | 101 |
| Mixture . | Clot time, min . | αTAT max rate, nM/min . | αTAT max level, nM . | αTAT max % of WB . |
|---|---|---|---|---|
| WB | 4:09 ± 0:19 | 47.2 ± 7.2 | 444 ± 49.6 | 100 |
| PRP + PPP | 7:54 ± 1:12 | 38 ± 4.9 | 267 ± 19.7 | 60 |
| PRP + buffy coat | 7:46 ± 1:35 | 32.4 ± 6.3 | 335 ± 53.7 | 75 |
| PRP + RBCs | 4:47 ± 0:25 | 48.4 ± 7.0 | 451 ± 51.4 | 101 |
The thrombin-generation parameters clot time, maximum (max) rate of αTAT formation, and max level of αTAT formation are presented. Data are presented as means ± SEM (n = 5).
The data in Tables 1 and 2 summarize the cellular sources of thrombin generation in each of the various reconstituted fractions. In PRP reconstituted with PPP, platelet counts were effectively normalized (1.08) while maintaining a minimum WBC contamination (0.08) and an undetectable number of RBCs (< 0.002); however, the clot time ratio was approximately double (1.86) that seen in WB. The addition of buffy coat to PRP slightly elevated the platelet ratio (1.22) and normalized the WBC ratio (1.05). The RBC ratio was slightly elevated (0.07) and the clot time ratio remained approximately doubled (1.83). PRP reconstituted with packed RBCs showed a slightly elevated platelet ratio (1.20) with some WBC contamination (0.19). The RBC ratio was increased to approximately 70% (0.68) and the clot time ratio was effectively normalized. From these data we can draw 4 conclusions: (1) insufficient reconstitution of platelets does not account for the longer clot times observed with PRP + PPP relative to WB, (2) WBC contamination in PRP does not account for the longer clot times observed with PRP + PPP relative to WB, (3) depletion of buffy coat does not prolong the clotting time (Table 1 PRP + RBC), and (4) buffy coat (Table 1 PRP + WBC) is not sufficient to normalize clotting times in the absence of RBCs. These data suggest that, in addition to platelets, RBCs play a major role in the initiation and propagation of thrombin generation in our model.
Washed RBCs
As the cell counts from the blood fractionations indicate, the crude fractionation procedures used did not yield pure cell populations. We therefore washed RBCs from CTI blood collected in the absence of other anticoagulants. Blood was drawn into CTI and centrifuged repeatedly at low speed to eliminate contaminating platelets and WBCs. Cell counts were performed before and after the washing procedure (Table 3) to analyze both the purity and integrity of the washed RBCs. This procedure effectively eliminated WBCs (< 0.1 × 103/μL) and platelets (< 0.1 × 103/μL) while maintaining the relative health of the RBCs. Blood smears exhibited < 1% neutrophil contamination. Compared with RBCs in the corresponding WB, washed RBCs maintained their overall shape (mean corpuscular volume, 86.7 vs 85 fL; mean corpuscular hemoglobin, 29.5 vs 29.3 pg) and hemoglobin concentrations (mean corpuscular hemoglobin concentration, 34 vs 34.5 g/dL). We further investigated the purity of the washed RBC populations using FACS, which indicated that RBC populations were > 99.9% positive for the RBC marker glycophorin A while being < 0.01% positive for the WBC marker CD45 or the platelet marker CD42b. Washed RBCs also showed an approximate 70% reduction in reticulocyte counts (CD 71) compared with the corresponding WB (supplemental Figure 1).
Properties of washed RBC populations
| Parameter . | Normal range . | WB . | Washed RBCs . |
|---|---|---|---|
| WBC, × 103/μL | 3.9-9.4 | 6.9 | < 0.1 |
| PLT, × 103/μL | 155-330 | 227 | < 0.1 |
| RBC parameters | |||
| RBC, × 106/μL | 4.14-5.52 | 4.65 | 5.08 |
| HGB, g/dL | 11.9-16.7 | 13.7 | 14.9 |
| MCV, fL | 83.2-96.0 | 86.7 | 85 |
| MCH, pg | 27.1-32.5 | 29.5 | 29.3 |
| MCHC, g/dL | 31-35.8 | 34 | 34.5 |
| Parameter . | Normal range . | WB . | Washed RBCs . |
|---|---|---|---|
| WBC, × 103/μL | 3.9-9.4 | 6.9 | < 0.1 |
| PLT, × 103/μL | 155-330 | 227 | < 0.1 |
| RBC parameters | |||
| RBC, × 106/μL | 4.14-5.52 | 4.65 | 5.08 |
| HGB, g/dL | 11.9-16.7 | 13.7 | 14.9 |
| MCV, fL | 83.2-96.0 | 86.7 | 85 |
| MCH, pg | 27.1-32.5 | 29.5 | 29.3 |
| MCHC, g/dL | 31-35.8 | 34 | 34.5 |
HGB indicates mean hemoglobin concentration; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; and MCHC, mean corpuscular hemoglobin concentration.
Blood smears showed < 1% neutrophils. Complete blood counts were performed on citrated samples of washed RBCs on an automated hematology analyzer. Cell counts were adjusted for the addition of 3.2% sodium citrate (9:1). Neutrophils were independently examined using a peripheral blood smear. Data were generated from a representative experiment.
FACS was also used to examine the surface expression of PS expression on RBCs in citrated WB and washed RBC fractions. PS expression on glycophorin A–positive RBCs was detected using FITC-annexin V (0.67%) and FITC-bovine lactadherin (0.4%). NEM and ionophore-treated RBCs bound FITC-labeled fXa in an fVa-dependent manner (Figure 5A-C), with FITC-fXa·fVa detected on approximately 0.6% of the RBC population (Figure 5D-E). These observations were consistently reproducible with either WB or washed RBCs and are consistent with the level reported in the healthy controls from other studies.12,18,19 Furthermore, the presence of fXa·fVa binding indicates that not only is a PS-equivalent surface present, but that this surface can support prothrombinase complex formation.
Prothrombinase binding to PS-expressing RBCs. WB was drawn via phlebotomy into 0.1 mg/mL of CTI, centrifuged at 150g, and then washed and treated with NEM and ionophore and the indicated reagent, as described in “Methods.” Treated cells were diluted to 2 × 106 cell/mL in RBC wash buffer and examined in a flow cytometer. Fluorescence gates were set using nonimmune IgG-FITC (A). PS exposure was detected with FITC-EGRck-fXa either in the absence (B) or presence (C) of saturating fVa. PS exposure was detected on untreated washed RBCs with FITC-EGRck-fXa/fVa (D [histogram] and E [scatter plot with PE-cd235a]).
Prothrombinase binding to PS-expressing RBCs. WB was drawn via phlebotomy into 0.1 mg/mL of CTI, centrifuged at 150g, and then washed and treated with NEM and ionophore and the indicated reagent, as described in “Methods.” Treated cells were diluted to 2 × 106 cell/mL in RBC wash buffer and examined in a flow cytometer. Fluorescence gates were set using nonimmune IgG-FITC (A). PS exposure was detected with FITC-EGRck-fXa either in the absence (B) or presence (C) of saturating fVa. PS exposure was detected on untreated washed RBCs with FITC-EGRck-fXa/fVa (D [histogram] and E [scatter plot with PE-cd235a]).
Thrombin generation on washed RBCs
A modification of the CTI blood model was used to examine the thrombin-generation potential of washed RBCs (Figure 6). For these assays, blood (n = 3) was drawn into CTI and the RBCs isolated and washed. Blood from the same subject was redrawn and CTI blood/PPP prepared. Three mixtures, WB, PPP, and PPP + washed RBCs, were activated with 5pM Tf and quenched over a 20-minute time course. WB (n = 3) clotted in 3:40 ± 0:18 minutes, generated αTAT at a maximum rate of 30 ± 3nM/min, and reached a maximum level of 354 ± 8.4nM αTAT (Figure 6A and Table 4). The corresponding PPP showed weak clots after an approximately 2-minute delay (5:35 ± 1:07 minutes) and exhibited minimal thrombin generation (maximum rate, 6 ± 0.9nM/min; maximum level, 79 ± 23nM). PPP reconstituted with washed RBCs to 40% hematocrit (equivalent to the initial WB) showed an improved clot time (4:10 ± 0:18 minutes) and increased αTAT generation (21 ± 2nM/min, 70% of WB) with a maximum αTAT of 242 ± 16nM (68% of WB). The data on αTAT generation in PPP + RBCs are consistent with the conclusion that RBCs are responsible for approximately 40% of αIIa generation in the corresponding WB when these data are corrected for the αTAT generation seen in PPP alone.
αTAT and mTAT ELISA analyses of PPP reconstituted with washed RBCs. WB was drawn via phlebotomy into 0.1 mg/mL of CTI, centrifuged at 150g, and subsequently washed as described in “Methods.” The same subject's blood was redrawn into 0.1 mg/mL of CTI and a fraction was centrifuged to obtain PPP. Three fractions: WB (○), PPP (▴), and PPP + washed RBCs (■), were then subjected to 5pM relipidated rTf. Samples were quenched at various intervals with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA (A) or mTAT ELISA (B). Data are shown as the means ± SEM (n = 3).
αTAT and mTAT ELISA analyses of PPP reconstituted with washed RBCs. WB was drawn via phlebotomy into 0.1 mg/mL of CTI, centrifuged at 150g, and subsequently washed as described in “Methods.” The same subject's blood was redrawn into 0.1 mg/mL of CTI and a fraction was centrifuged to obtain PPP. Three fractions: WB (○), PPP (▴), and PPP + washed RBCs (■), were then subjected to 5pM relipidated rTf. Samples were quenched at various intervals with EDTA and Phe-Pro-Arg chloromethylketone and analyzed via αTAT ELISA (A) or mTAT ELISA (B). Data are shown as the means ± SEM (n = 3).
Thrombin-generation parameters from analyses of reconstituted PPP time courses
| Mixture . | Clot time, min . | Max rate, nM/min . | Max level, nM . |
|---|---|---|---|
| αTAT parameters | |||
| WB | 3:40 ± 0:18 | 30.1 ± 2.9 | 354 ± 8.42 |
| PPP | 5:35 ± 1:07 | 6.0 ± 0.9 | 79 ± 23 |
| PPP + RBCs | 4:10 ± 0:18 | 21.1 ± 2.0 | 242 ± 16 |
| mTAT parameters | |||
| WB | 3:40 ± 0:18 | 1.2 ± 0.8 | 14.7 ± 11.3 |
| PPP | 5:35 ± 1:07 | 0.4 ± 0.2 | 4.1 ± 2.5 |
| PPP + RBCs | 4:10 ± 0:18 | 0.7 ± 0.2 | 9.1 ± 2.5 |
| Mixture . | Clot time, min . | Max rate, nM/min . | Max level, nM . |
|---|---|---|---|
| αTAT parameters | |||
| WB | 3:40 ± 0:18 | 30.1 ± 2.9 | 354 ± 8.42 |
| PPP | 5:35 ± 1:07 | 6.0 ± 0.9 | 79 ± 23 |
| PPP + RBCs | 4:10 ± 0:18 | 21.1 ± 2.0 | 242 ± 16 |
| mTAT parameters | |||
| WB | 3:40 ± 0:18 | 1.2 ± 0.8 | 14.7 ± 11.3 |
| PPP | 5:35 ± 1:07 | 0.4 ± 0.2 | 4.1 ± 2.5 |
| PPP + RBCs | 4:10 ± 0:18 | 0.7 ± 0.2 | 9.1 ± 2.5 |
Thrombin- and meizothrombin-generation parameters are from Figure 6. The clot time, maximum (max) rate of αTAT and mTAT formation and max level of αTAT and mTAT formation were calculated from the αTAT and mTAT ELISA data. Data are shown as the means ± SEM (n = 3).
mIIa generation was also assessed using the mTAT ELISA (Figure 6B). WB generated mTAT at a rate of 1.2 ± 0.8nM/min and reached a maximum level of 14.7 ± 11.3nM. PPP reconstituted to a 40% hematocrit showed significant mTAT formation (Table 4) compared with the corresponding WB, with a maximum rate of approximately 0.7 ± 0.2nM/min (approximately 58% of WB) and reaching a level of 9.1 ± 2.9nM (approximately 62% of WB). PPP alone showed a lesser amount of mTAT generation (maximum rate, 0.4 ± 0.2nM/min; maximum level, 4.4 ± 2.5nM, 27% of WB).
Prothrombinase assembly on washed RBCs
The ability of washed RBCs to support IIase was evaluated (Figure 7A). Washed RBCs were preincubated with fVa and fXa before initiation of the reaction with prothrombin and AT. Samples were quenched at desired intervals as with WB samples and thrombin generation was assessed via αTAT ELISA. Controls consisted of 250nM PCPS, no exogenous lipid, and RBC centrifugation supernatant. The RBC centrifugation supernatant that was added was equivalent to the contaminating fraction of buffer present in the 40% RBC hematocrit and would contain any exogenous lipids or microparticles in the RBC preparation. Experiments containing 250nM PCPS showed an immediate burst of αTAT generation, a maximum rate of 31nM/min, and a maximum level of αTAT of 128nM. Experiments performed in the absence of exogenous lipid or the presence of the RBC supernatant showed no αTAT formation. FXa·fVa incubated with RBCs at a 40% hematocrit showed a brief (approximately 2 minutes) lag phase, followed by a significant and steady level of αTAT generation (14nM/min), reaching a maximum of approximately 119nM. This level of thrombin generation corresponds to approximately 18.3pM IIase. The FACS data suggested that approximately 0.6% of RBCs support fXa·fVa binding (Figure 5). These data translate to roughly 550 IIase sites per PS-positive RBC. When experiments were performed on RBCs pretreated with a molar excess (40nM) of unlabeled bovine lactadherin, the ability of these cells to support prothrombinase was nearly abolished (< 3%).
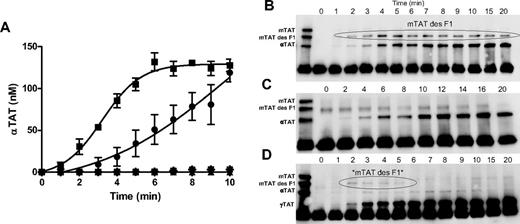
Thrombin-generation potential of RBCs. WB was drawn via phlebotomy into 0.1 mg/mL of CTI and washed as described in “Methods.” Washed RBCs were counted and stored at 37°C. (A) IIase (20nM fVa, 200pM fXa final concentrations) was assembled in samples containing 250nM PCPS (■), 40% washed RBCs (●), RBCs pretreated with 40nM bovine lactadherin (*), RBC wash supernatant (▾), or no exogenous surface (▴), and the reactions were initiated with a solution containing prothrombin and AT (1.4 and 3.4μM final concentrations, respectively). Aliquots were removed at selected time points, quenched with EDTA and Phe-Pro-Arg chloromethylketone, and the soluble fraction was analyzed via αTAT ELISA and Western blotting. Data are shown as the means ± SEM (n = 5). Western blotting was performed on nonreduced samples probing with the burro anti-AT polyclonal Ab. Lane 1 shows the mTAT/mTAT des F1/αTAT standard; lanes 2-, 0- to 20-minute time-course samples. (B) Representative Western blot from PCPS prothrombinase time course. (C) Representative Western blot from RBC prothrombinase time course. (D) Representative Western blot from a PMA-treated RBC prothrombinase time course.
Thrombin-generation potential of RBCs. WB was drawn via phlebotomy into 0.1 mg/mL of CTI and washed as described in “Methods.” Washed RBCs were counted and stored at 37°C. (A) IIase (20nM fVa, 200pM fXa final concentrations) was assembled in samples containing 250nM PCPS (■), 40% washed RBCs (●), RBCs pretreated with 40nM bovine lactadherin (*), RBC wash supernatant (▾), or no exogenous surface (▴), and the reactions were initiated with a solution containing prothrombin and AT (1.4 and 3.4μM final concentrations, respectively). Aliquots were removed at selected time points, quenched with EDTA and Phe-Pro-Arg chloromethylketone, and the soluble fraction was analyzed via αTAT ELISA and Western blotting. Data are shown as the means ± SEM (n = 5). Western blotting was performed on nonreduced samples probing with the burro anti-AT polyclonal Ab. Lane 1 shows the mTAT/mTAT des F1/αTAT standard; lanes 2-, 0- to 20-minute time-course samples. (B) Representative Western blot from PCPS prothrombinase time course. (C) Representative Western blot from RBC prothrombinase time course. (D) Representative Western blot from a PMA-treated RBC prothrombinase time course.
Time-course samples from prothrombin activation studies on PCPS and RBCs (Figure 7A) were analyzed via Western blotting. Figure 7B displays the formation of TAT species over a prothrombinase time course on PCPS vesicles. The formation of mTAT des fragment 1 appears at 2 minutes and reaches a maximum level in 4 minutes and declines over the rest of the time course. The appearance of αTAT is coincident with mTAT reaching a stable level at approximately 7 minutes. However, when RBCs are used as the surface to support prothrombin activation (Figure 7C), the predominant form produced across the time course is αTAT. mTAT is not apparent in the Western blot and the mTAT ELISA detected no significant levels (< 2nM). When RBCs were treated with PMA (Figure 7D), which has been demonstrated to dose dependently increase PS expression in RBCs (supplemental Figure 2),20 transient levels of mTAT des fragment 1 and αTAT were observed. However, the dominant and accumulating TAT species appeared to be that of γTAT, which is detected by the αTAT but not mTAT ELISA. Therefore, the use of PMA to increase RBC prothrombin activation in this system results in an environment in which both αTAT and mTAT and their respective enzyme species are unstable.
Discussion
We developed αTAT and mTAT ELISAs to examine the pathway of prothrombin activation in Tf-activated WB. In contrast to commercial assays, which measure total TAT, these assays independently recognize mTAT and αTAT. Using these assays, the production of αTAT and mTAT was measured during Tf-activated coagulation. mTAT production occurred concurrently with αTAT formation, reaching approximately 4.4% of the observed αTAT. When the relative distributions of αTAT and mTAT were examined in a larger population, the level of αTAT was relatively constant within subjects, with variability between subjects, which is consistent with previous reports.14 However, mTAT was variable both within and between subjects, which suggests that additional factors influence mTAT measurements in clotting blood. Analyses of the stability of TAT species in WB support this concept, with mTAT species appearing to be both degraded and adsorbed to cellular surfaces. Interestingly, when washed RBCs are artificially induced to express more PS, both the mTAT and αTAT forms are unstable, suggesting that under these conditions, RBCs secrete an active protease that converts both forms to γTAT.
Computational analyses based on the behavior of prothrombinase on phospholipid vesicles predicted that the amount of prothrombin processed through the mIIa pathway would be approximately 9 times the amount of mTAT observed. When the mTAT data from 10 subjects were interpreted using this conversion factor, approximately 27% (104nM) of the αTAT (383nM) measured was derived from the mIIa pathway. In light of the recent report demonstrating that prothrombin activation on platelets does not generate detectable mIIa,6 our confirmation of the previous report of mIIa in clotting WB8 raised the question of the source of this product. RBCs and WBCs exist in sufficient abundance to potentially contribute to this phenomenon.21 WBCs have been reported to display a procoagulant phenotype in the context of ischemic vascular disease22 and sepsis via expression of Tf.23-25 Enzymatic complexes bound to monocytes and neutrophils exhibit IIase-binding values similar to the platelet-bound complex; however, complexes bound to lymphocytes are only 25% as active.26,27 RBCs make up most of the cellular fraction of blood, comprising 35%-45% by volume.21 It has been long known that there is an association between hematocrit levels and thrombosis.28-30 Furthermore, previous reports suggest that the ensuing thrombotic complications may be a result of biochemical and biomechanical forces.31-34
We developed a rapid fractionation procedure for CTI blood in the absence of other anticoagulants to yield minimally altered preparations of RBCs, PRP, PPP, and buffy coat that could be challenged in isolation or combination by Tf to investigate the contributions of each cellular surface to thrombin generation. Our data show that RBCs play a central role in thrombin generation in Tf-activated WB. Previous blood-fractionation studies have used chelating anticoagulants, which have been shown to alter coagulation biochemistry.35 In the current approach, CTI WB is rapidly fractionated into usable components in the absence of exogenous chelating or heparinoid anticoagulants. This fractionation technique provides a source of relatively unadulterated blood cells that can be used to study the cell biology of thrombin generation in vitro. After fractionation, PRP displayed a significantly attenuated thrombin generation potential compared with its corresponding WB. The addition of buffy coat to PRP had some effect on thrombin generation, whereas the addition of RBCs normalized the clot time, rate, and extent of TAT formation. Cell counts in the reconstituted blood mixtures rule out other contaminating cell types as the culprit for these phenomena.
When washed RBCs were added to PPP, the data showed that in the absence of exogenous phospholipid sources, RBCs were capable of producing αTAT at approximately half the rate and extent seen in the corresponding WB. This result is consistent with the increased αTAT observed when PRP was reconstituted with RBCs. More importantly, these cells were capable of generating mTAT, which addresses the question of where mIIa generation can occur. The low levels of thrombin generation observed in PPP can most likely be attributed to the presence of plasma lipoproteins,36 microparticles,37 and the low (25nM) level of phospholipid presented with our Tf reagent.
PS expression in CTI WB was measured via FACS using annexin V and bovine lactadherin. Our results indicate that approximately 0.6% of all RBCs showed some level of PS presentation on their surface, which is consistent with previous reports.12,18,19 More significantly, we observe an equivalent level of fVa-dependent fXa binding to the RBC population, which is consistent with the conclusion that some RBCs support IIase. Subsequent IIase assays performed on washed RBCs displayed significant thrombin generation. An initial kinetic study of this system suggests approximately 550 IIase sites per RBC assuming a 0.6% PS-positive population. Given the relative abundance of RBCs in blood, this represents a significant (approximately 3 × 107/mL) population of IIase-supporting cells. Interestingly, IIase experiments performed on αIIa-treated RBCs showed an increase in activity. This observation suggests that the thrombin-generation potential of this relatively small fraction of RBCs may be amplified when αIIa is abundant. A possible candidate for these phenomena are reticulocytes, which make up approximately 1% of the RBC population.38 However, the washed RBCs were significantly reduced (< 30%) in this cell type, suggesting that another population of PS-expressing RBCs is the source of PS. Phospholipid asymmetry is one of the competing theories for the clearance of senescent RBCs, whereby PS is detected by macrophages in the spleen, leading to erythrophagocytosis.39,40 At any given time in a subject's blood, approximately 0.1%-1% of RBCs are senescent,41 a value coincident with the PS expression data in the present study and previous studies.12,18,19 It is therefore conceivable that these cells are responsible for the thrombin generation seen in our assays.
In addition to the identification of the source of mIIa production in WB, the results of the present study support a biochemical role for RBCs in coagulation. In Tf-activated blood, RBCs and platelets both appear to play significant roles in thrombin generation.
The online version of this article contains a data supplement.
Presented in part at the 53rd ASH Annual Meeting and Exposition, December 10-13, 2011, San Diego, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ruhin Yuridullah for his contributions to αTAT and mTAT ELISA assay development and Dr Mohandas Narla for advice.
This research was supported by National Institutes of Health grant P01HL46703 to K.G.M. (project 1).
National Institutes of Health
Authorship
Contribution: M.F.W. performed all experiments and wrote the manuscript; V.Z. performed all ELISA assays and helped write the manuscript; and T.O. and K.G.M. participated in experimental design and helped write the manuscript.
Conflict-of-interest disclosure: K.G.M. is a consultant for Daiichi-Sankyo, Merck, Baxter, GTI, Alnylam, and T2 Biosystems and chairman of the board of Haematologic Technologies. The remaining authors declare no competing financial interests.
Correspondence: Kenneth G. Mann, 208 South Park Dr, CRF Room 235, Colchester, VT 05446; e-mail: kenneth.mann@uvm.edu.


![Figure 5. Prothrombinase binding to PS-expressing RBCs. WB was drawn via phlebotomy into 0.1 mg/mL of CTI, centrifuged at 150g, and then washed and treated with NEM and ionophore and the indicated reagent, as described in “Methods.” Treated cells were diluted to 2 × 106 cell/mL in RBC wash buffer and examined in a flow cytometer. Fluorescence gates were set using nonimmune IgG-FITC (A). PS exposure was detected with FITC-EGRck-fXa either in the absence (B) or presence (C) of saturating fVa. PS exposure was detected on untreated washed RBCs with FITC-EGRck-fXa/fVa (D [histogram] and E [scatter plot with PE-cd235a]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/18/10.1182_blood-2012-05-427856/4/m_zh89991298490005.jpeg?Expires=1769264885&Signature=cMJ4KBXd6vgHpc9HtOygVaRIkIrl58CLEy4SguAvIgi4STcsl3uxXSR2Qdv3sOHYOH0-LjDa3VB9fFmDAdU5W5t4vYw3qvYP3a3rVKCp0ppGxFiNFNpyw0kacb4bZBhYcjefIRTHUG77qmGLvymPk9imrQ1ToSDqf4XX3b-VVcnpP7xM~QBeXZM364rXT~6bMPKT9WicEsJ72I6ZovFuVwzI8HMmtaY0ChGpUBhKMwpVDF2U8pzLfXLDJLbg~2blGYTDoJNHod5dyS28haJaoWAt1mpGISMAI~48HzxAO5z-OxJNWtFMnHTKmleJVGB42-vCgJsP5IUs1lV4z9gQQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)