To the editor:
Sickle cell disease (SCD) is a hemoglobinopathy caused by a single amino acid change in the β globin subunit that predisposes hemoglobin to abnormal polymerization leading to stiff, sickled erythrocytes, and microvascular occlusions.1 Although the vascular consequences of SCD are triggered by changes in the erythrocyte,2 a chronic inflammatory state often ensues with many other cell types contributing to SCD pathology. For example, sickling of erythrocytes in the microvasculature is accompanied by increased platelet and leukocyte-endothelial (L-E) interactions that are strongly associated with vascular complications3 including stroke, priapism, leg ulcers, and nephropathy.4 Therapeutic targeting of molecules responsible for mediating these adhesive interactions has been shown to be potentially beneficial in preclinical models of SCD.5 Inhibition of P-selectin in particular has been associated with reduced L-E interactions in mouse models of SCD.6 The leukocyte ligand for P-selectin is P-selectin glycoprotein ligand-1 (Psgl-1), which also interacts with E- and L-selectin.
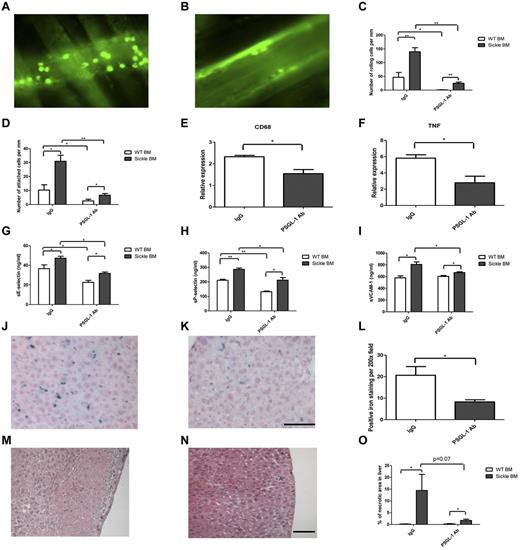
Psgl-1 inhibition may be particularly effective in reducing adhesive interactions in SCD since Psgl-1–mediated signaling also affects activation of leukocytes,7 which in turn affects adhesive properties of the endothelium.8 To determine the effectiveness of Psgl-1 inhibition on reduction of L-E interactions in the microvasculature, we studied a humanized mouse model of SCD9 treated with an anti–mouse Psgl-1 antibody. First, C57BL/6J mice underwent a bone marrow transplantation (BMT) procedure as previously described.8 Recipient mice received bone marrow from wild-type donors (Hbb+/+) or donors homozygous for the sickle cell mutation (Hbbhβs/hβs). Eight weeks after BMT, mice receiving Hbbhβs/hβs marrow (n = 10) were anemic compared with mice receiving Hbb+/+ marrow (n = 10; hematocrit - 28.9 ± 1.0 vs 36.7 ± 0.8%, P < .0001), (hemoglobin - 10.0 ± 0.5 vs 12.3 ± 0.4 g/dL, P < .001) and (RBC counts 7.5 ± 0.5 vs 9.1 ± 0.2 × 106/μL, P < .01). Sickle-shaped erythrocytes were frequent in the peripheral blood smears of Hbbhβs/hβs mice and Hbbhβs/hβs mice exhibited a marked reticulocytosis compared with Hbb+/+ mice (reticulocyte - 30.2 ± 4.4 vs 3.4 ± 0.6% of RBCs, P < .00001). At the time of sacrifice (20 weeks after BMT), the average spleen size of Hbbhβs/hβs mice was 370.7 ± 55.4 mg compared with 74.9 ± 2.4 mg in Hbb+/+ mice, P < .000001. At 16 weeks post BMT, mice were treated with weekly IV injection of anti–Psgl-1 antibody or control IgG antibody for 4 weeks. Intravital microscopy8 was performed 20 weeks following BMT using intravenous rhodamine to label leukocytes. Leukocyte rolling and firm attachment were increased in Hbbhβs/hβs mice compared with Hbb+/+ mice. However, anti–Psgl-1 treatment completely reversed the increased leukocyte rolling and firm attachment within the Hbbhβs/hβs mice. (Figure 1A-D). Reduced L-E interactions were associated with reductions in levels of circulating soluble E-selectin (sE-sel), soluble P-selectin (sP-sel), and soluble vascular cell adhesion molecule 1(sVCAM-1; Figure 1G-I). Four weeks of treatment was sufficient to reduce iron deposition and necrotic areas of liver (Figure 1L,O). This was associated with reduced CD68 and TNF-α expression in the liver by RT-PCR (Figure 1E-F). In conclusion, inhibition of Psgl-1 may be an effective treatment for reducing vascular complications of SCD.
Effect of Psgl-1 antibody treatment on SCD. Representative intravital microscopy images of venules from Hbbhβs/hβs BMT mice that received either IgG (A) or PSGL-1 (B) Ab injections. Number of rolling (C) or attached (D) cells per mm. Transcription level of CD68 (E) and TNF-α (F) of liver tissues. Panels G-I are levels of circulating adhesion molecules, (sE-sel, sP-sel, and sVCAM-1). Representative micrographs of liver sections from Hbbhβs/hβs BMT mice receiving IgG, (J,M); or PSGL-1 Ab injection (K,N). Panels J and K are iron staining, panels M and N are H&E staining. (L) Quantification of iron deposition and (O) % of necrotic area from liver sections. *P < .05. **P < .001. N = 4-5 per group. Magnification is 200×, bar = 100 μm.
Effect of Psgl-1 antibody treatment on SCD. Representative intravital microscopy images of venules from Hbbhβs/hβs BMT mice that received either IgG (A) or PSGL-1 (B) Ab injections. Number of rolling (C) or attached (D) cells per mm. Transcription level of CD68 (E) and TNF-α (F) of liver tissues. Panels G-I are levels of circulating adhesion molecules, (sE-sel, sP-sel, and sVCAM-1). Representative micrographs of liver sections from Hbbhβs/hβs BMT mice receiving IgG, (J,M); or PSGL-1 Ab injection (K,N). Panels J and K are iron staining, panels M and N are H&E staining. (L) Quantification of iron deposition and (O) % of necrotic area from liver sections. *P < .05. **P < .001. N = 4-5 per group. Magnification is 200×, bar = 100 μm.
Authorship
Acknowledgments: This work was supported by the National Institutes of Health (HL073150 to D.T.E.) and a VA Merit Award (BX000353 to D.T.E.).
Contribution: W.L. designed research, performed research, analyzed data, and wrote the paper; A.C. and D.T.E. designed research and wrote the paper; and H.W., C.G., K.B., and J.W. performed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel T. Eitzman, MD, University of Michigan, Cardiovascular Research Center, 7301A MSRB III, 1150 W Medical Center Dr, Ann Arbor, MI 48109-0644; e-mail: deitzman@umich.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal