Abstract
Dense granules are important in platelet aggregation to form a hemostatic plug as evidenced by the increased bleeding time in mice and humans with dense granule deficiency. Dense granules also are targeted by antiplatelet agents because of their role in thrombus formation. Therefore, the molecular understanding of the dense granule and its biogenesis is of vital importance. In this work, we establish a human megakaryocytic cell line (MEG-01) as a model system for the study of dense granule biogenesis using a variety of cell biology and biochemical approaches. Using this model system, we determine the late endocytic origin of these organelles by colocalization of the internalized fluid phase marker dextran with both mepacrine and transmembrane dense granule proteins. By mistargeting of mutant dense granule proteins, we demonstrate that sorting signals recognized by adaptor protein-3 are necessary for normal transport to dense granules. Furthermore, we show that tissue-specific Rab32 and Rab38 are crucial for the fusion of vesicles containing dense granule cargo with the maturing organelle. This work sheds light on the biogenesis of dense granules at the molecular level and opens the possibility of using this powerful model system for the investigation of new components of the biogenesis machinery.
Introduction
Platelets contribute to normal hemostasis by releasing their α granule (AG) and dense granule (DG) components at sites of vascular injury. DGs concentrate small molecules such as serotonin, ADP, and calcium, and their involvement in hemostasis is evident in patients presenting with bleeding disorders because of deficiency of these granules.1,2 In contrast, DG biogenesis and secretion have been identified as targets for antithrombotic drugs.3-5 Despite the importance of DGs for human health, very little is known about their biogenesis. DGs are synthesized in the bone marrow by megakaryocytes (MKs). These cells are difficult to isolate, culture, and manipulate, which explains this knowledge gap. Thus, the lack of convenient systems to study DG formation at the cellular and molecular level has been a major limitation, leaving the mechanism involved in biogenesis unclear.
Unlike most secretory granules produced in other cell types, DGs may not originate from the trans-Golgi network. Instead, the biogenesis of DGs may involve a specialized biosynthetic mechanism that connects the secretory and the endocytic pathways6,7 (Figure 1A). Consistent with that notion, one study suggested a possible multivesicular body (MVB)/late endosome origin for DGs using granulophysin as a DG marker.8 However, granulophysin was later discovered to be the same molecule as CD63/LAMP3 that localizes to several organelles in addition to DGs.9 Therefore, the MVB/late endosome origin of dense granules remains an open question.
Platelet DGs belong to a group of lysosome-related organelles (LROs) that also includes melanosomes in melanocytes. Defects in genes and proteins involved in LRO biogenesis have been identified in Hermansky-Pudlak syndrome (HPS) patients and animal models of the disease that present with a combination of albinism and prolonged bleeding because of abnormal melanosomes and DGs.1 Examples of HPS proteins are adaptor protein-3 (AP-3) and Rab38. AP-3 operates at early endosome–associated tubules where it recognizes both tyrosine-based and dileucine-based sorting signals in the cytosolic tail of transmembrane protein cargo and packages them into transport vesicles destined to lysosomes and melanosomes.10-13 It is possible that similar sorting signals and AP-3–dependent mechanism operate in a transport pathway from early endosome–associated tubules to maturing DGs (Figure 1A). This hypothesis has not yet been tested experimentally.
Rab proteins are key regulators of vesicular trafficking that mediate vesicle motility, tethering, and fusion within the secretory and endocytic pathways.14 Mutation of Rab38 in rodent disease models cause DG and melanosome deficiency.15,16 Rab38 and its very close homolog Rab32 cooperate in melanosome biogenesis, at least in part working through the AP-3 pathway.17,18 It is possible that Rab32 also participates in DG biogenesis and that both Rabs cooperate with AP-3 in the delivery of transmembrane proteins to the maturing DG. However, the cellular location and molecular function of Rab32 and Rab38 in DG biogenesis are unknown.
Here, we show that the megakaryocytic cell line MEG-01 is a very good model system that recapitulates what is known about DGs and allows various experimental approaches to study DG biogenesis. Using this system, we established the MVB/late endosome origin for the organelle. We demonstrate that DG transmembrane proteins depend on tyrosine-based and dileucine-based sorting signals for normal transport to DGs. We also show that Rab32 and Rab38 likely define a biosynthetic transport pathway from early endosome–associated tubules to maturing DGs and that they have a key role in vesicle tethering, fusion with the maturing organelle, or both.
Methods
Antibodies
List of primary antibodies used in the study
| Target . | Host . | Type . | Company/Laboratory . |
|---|---|---|---|
| Rab32 | Rabbit | Polyclonal | Di Pietro |
| Rab38 | Rabbit | Polyclonal | Di Pietro |
| AP-3 δ (SA4) | Mouse | Monoclonal | Peden11 |
| LAMP2 | Mouse | Monoclonal | Santa Cruz Biotechnology |
| PF-4 | Goat | Polyclonal | Santa Cruz Biotechnology |
| MRP4 (IF) | Rat | Monoclonal | Alexis Biochemicals |
| MRP4 (IB) | Mouse | Monoclonal | Abnova |
| Clathrin | Mouse | Monoclonal | Abcam |
| vWF | Rabbit | Polyclonal | Dako |
| Rab7a | Rabbit | Monoclonal | Cell Signaling Technology |
| α-tubulin | Mouse | Monoclonal | Sigma |
| Target . | Host . | Type . | Company/Laboratory . |
|---|---|---|---|
| Rab32 | Rabbit | Polyclonal | Di Pietro |
| Rab38 | Rabbit | Polyclonal | Di Pietro |
| AP-3 δ (SA4) | Mouse | Monoclonal | Peden11 |
| LAMP2 | Mouse | Monoclonal | Santa Cruz Biotechnology |
| PF-4 | Goat | Polyclonal | Santa Cruz Biotechnology |
| MRP4 (IF) | Rat | Monoclonal | Alexis Biochemicals |
| MRP4 (IB) | Mouse | Monoclonal | Abnova |
| Clathrin | Mouse | Monoclonal | Abcam |
| vWF | Rabbit | Polyclonal | Dako |
| Rab7a | Rabbit | Monoclonal | Cell Signaling Technology |
| α-tubulin | Mouse | Monoclonal | Sigma |
IF indicates immunofluorescence; and IB, immunoblotting.
List of secondary antibodies used in the study
| Reactivity . | Conjugation . | Company . |
|---|---|---|
| Rat, rabbit, mouse | Alexa-488, -546, -647 | Invitrogen |
| Goat | HRP | Invitrogen |
| Rabbit | 12-nm gold | Jackson ImmunoResearch Laboratories |
| Mouse | 18-nm gold | Jackson ImmunoResearch Laboratories |
| Rabbit, mouse | HRP | GE Healthcare |
| Reactivity . | Conjugation . | Company . |
|---|---|---|
| Rat, rabbit, mouse | Alexa-488, -546, -647 | Invitrogen |
| Goat | HRP | Invitrogen |
| Rabbit | 12-nm gold | Jackson ImmunoResearch Laboratories |
| Mouse | 18-nm gold | Jackson ImmunoResearch Laboratories |
| Rabbit, mouse | HRP | GE Healthcare |
Cloning of VMAT2 and LAMP2 cDNAs
The cDNAs for VMAT2 and LAMP2A were amplified from total RNA of MEG-01 cells by reverse transcriptase-PCR and subsequently cloned in-frame into pEGFP and pmCherry.
Cell culture
MEG-01 cells were obtained from ATCC and cultured in RPMI-1640 supplemented with 10% (v/v) fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.3 μg/mL glutamine. Mouse bone marrow MKs were isolated by a 4-step BSA density gradient followed by a continuous Ficoll velocity gradient as described previously.19
MEG-01 cells were transfected using the Nucleofector electroporation system (Lonza). For siRNA treatments, 2 sequential transfections were performed on days 1 and 4; cells were analyzed on day 7. Oligonucleotides (Sigma) used for siRNA are as follows: negative control (SIC001-10 NMOL), Rab32 (SASI_Hs02_00342400), Rab38 (SASI_Hs01_00247037). For dextran uptake experiments, cells were incubated for 16 hours at 37°C in medium containing 250 μg/mL dextran Alexa Fluor 647 or Oregon Green 488 BAPTA-1 dextran (Invitrogen) followed by a 4-hour chase period in medium lacking dextran. Mepacrine was added to cells to a final concentration of 10μM followed by a 5-minute incubation at 37°C before imaging.
Electron microscopy
Cells grown on Aclar (Ted Pella) were subsequently fixed in 2% electron microscopy grade glutaraldehyde in phosphate-buffered saline (PBS) for 45 minutes, washed in PBS, postfixed in 1% aqueous osmium tetroxide for 15 minutes, and then washed in distilled water (dH2O). The cells were dehydrated in an ethanol series and flat embedded in LR White resin. Groups of cells were cut out, mounted, and 90-nm sections were collected, stained with 2% aqueous uranyl acetate for 15 minutes, poststained in Reynold's lead stain, and viewed on a 2000 transmission electron microscope (JEOL). High-pressure freezing (HPF) was performed as described previously.20 For immunogold labeling, 90-nm sections were collected on nickel slot grids, blocked with 20% goat serum in PBS, blotted, and then incubated in the primary antibodies overnight at 4°C. Grids were washed in PBS-Tween 20 and incubated in the secondary antibodies for 1.5 hours. Grids were washed first in PBS-Tween 20 and then in PBS, fixed in 0.5% glutaraldehyde in PBS, washed in dH2O, and dried. The grids were stained with 2% aqueous uranyl acetate in 70% methanol/30% water for 7 minutes, rinsed in 70% methanol/30% water, dried, and then poststained in Reynold's lead stain, rinsed in dH2O, and dried. Images were taken on a 2000 transmission electron microscope (JEOL).
Confocal fluorescence microscopy
For the live cell imaging experiments, cells were plated in glass-bottomed 35-mm dishes, and phorbol 12-myristate 13-acetate was added to a final concentration of 10nM 24 hours before imaging. The samples were imaged using a temperature-controlled chamber at 37°C and 5% CO2 on an IX81 spinning-disk confocal fluorescence microscope (Olympus). Fixation and immunofluorescence staining were performed as described previously.13 Immunofluorescence microscopy samples were examined using the same microscope utilized for the live cell fluorescence imaging experiments. Fixed and live cell sample images were acquired and analyzed in Slidebook 5.0 software (Intelligent Imaging Innovations). The colocalization module with auto threshold was used to determine the Manders' overlap coefficient21 (MOC) of dual-color images processed with both Gaussian and Laplacian 2-dimensional filters. MOC was used to determine the level of colocalization between marker pairs that are both membrane-bound or both contained inside the organelles (luminal markers). For colocalization of a membrane-bound marker with a luminal marker, the percentage of structures containing both markers was determined.
Subcellular fractionation
A postnuclear supernatant was prepared by homogenizing MEG-01 cells with a dounce homogenizer in buffer H (20mM Hepes pH 7.4) containing 0.32M sucrose and protease inhibitors followed by centrifugation for 20 minutes at 800g at 4°C. The postnuclear supernatant (250 μL) was loaded onto a 12-mL linear sucrose gradient (10%-60%) in buffer H. The sample was centrifuged at 113 000g for 6 hours in a SW41Ti rotor in an L8-70M ultracentrifuge (Beckman Coulter) at 4°C. Fractions of 1 mL were collected and used for immunoblotting, immunoprecipitation, ADP determination, and both Alexa Fluor 647 and Oregon Green 488 BAPTA-1 dextran fluorescence intensity reading.
Biochemical procedures
For immunoblotting, proteins were fractionated on precast 4% to 20% gradient SDS/polyacrylamide gels (Invitrogen) and transferred by electroblotting to polyvinylidene difluoride membranes. Membranes were incubated sequentially with blocking buffer, primary antibody, and horseradish peroxidase–conjugated secondary antibody as described previously.22 Bound antibodies were detected using ECL Prime Western blotting reagent (GE Healthcare). Immunoprecipitations were carried out using protein G magnetic beads (Millipore) and 2 μg of the appropriate antibody. ADP was determined by bioluminescence using the Enzylight ADP assay kit (BioAssay Systems). The fluorescence intensity of dextran Alexa Fluor 647 and Oregon Green 488 BAPTA-1 dextran in the sucrose gradient samples was measured using a microplate reader Victor3V (PerkinElmer Life and Analytical Sciences).
Results
MEG-01 cells provide a very good model system to study DG biogenesis
MEG-01 cells display the typical markers of differentiated megakaryocytes, generate AGs and DGs, produce platelet-like particles, and seem to resemble primary megakaryocytes better than other cell lines.23-27 We corroborated MEG-01 cells and primary megakaryocytes isolated from mouse bone marrow express surface proteins such as CD41 to a similar extent (data not shown). We then carried out several experiments to confirm the presence of DGs and various markers in MEG-01 cells. First, the cells were subjected to HPF and processed for thin-section electron microscopy. Of the different approaches tested, samples fixed or embedded with glutaraldehyde-uranyl acetate-Lowicryl HM20 worked best for overall preservation of membrane-bound structures. Based on the morphology and content of internal dense material, MEG-01 cells showed the presence of mature DGs together with a large number of immature DGs and MVBs (Figure 1B and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Quantitative analysis of electron micrographs of 19 MEG-01 cells showed they contain 0.6 ± 0.1 mature DG/10 μm2, 1.8 ± 0.3 immature DG/10 μm2, and 1.6 ± 0.2 MVB/10 μm2, whereas a similar analysis of 18 primary megakaryocytes isolated from mouse bone marrow showed 2.5 ± 0.4 mature DG/10 μm2, 0.5 ± 0.1 immature DG/10 μm2, and 1.0 ± 0.2 MVB/10 μm2 (970 organelles counted). Second, the DG integral membrane protein markers MRP4 (ADP transporter) and LAMP228,29 were detected by immunofluorescence microscopy in fixed MEG-01 cells (Figure 1C and supplemental Figure 2). MRP4 and LAMP2 display very good colocalization (MOC = 0.63 ± 0.06) consistent with previous findings in platelet DGs. Third, LAMP2 and VMAT2 (the putative DG serotonin transporter)30 were amplified from MEG-01 mRNA; cloned into pEGFP and pmCherry, respectively; and transfected into MEG-01 cells. Live cell spinning-disk confocal fluorescence microscopy show LAMP2-GFP and VMAT2-Cherry localize to the same structures (MOC = 0.58 ± 0.03), indicating the tagged proteins are correctly targeted to DGs (Figure 1D and supplemental Video 1). These levels of colocalization were significantly higher than those obtained in control experiments using LAMP2 and peroxisomal markers (MOC = 0.08 ± 0.01 or 0.09 ± 0.01) or MRP4 and LAMP2 with the AG marker VWF (MOC = 0.12 ± 0.01 and 0.18 ± 0.01, respectively; supplemental Figure 3). Fourth, live MEG-01 cells expressing VMAT2-Cherry were incubated with the DG lumen marker mepacrine and imaged by confocal fluorescence microscopy (Figure 2A). Mepacrine and VMAT2-Cherry largely colocalize in MEG-01 cells (95% ± 2% of the mepacrine structures contain both markers), corroborating both the presence of DGs and VMAT2-Cherry targeting to these organelles. Similar results were obtained when using LAMP2-Cherry and mepacrine (supplemental Figure 4B). Furthermore, MEG-01 cells dense granules labeled with LAMP2-GFP fused with the plasma membrane on stimulation with 1 U/mL thrombin, showing MEG-01 cells produce functional dense granules (supplemental Figure 5).
MEG-01 cells have DGs that can be studied by different microscopy techniques. (A) Model depicting the protein traffic to DG. DG transmembrane proteins (gold) follow the secretory pathway through the Golgi complex to early endosomes where they are selectively targeted to maturing DGs originated from MVBs. (B) Thin-section transmission electron microscopy image of a MEG-01 cell subjected to HPF, fixed or embedded with glutaraldehyde-uranyl acetate-Lowicryl HM20. Original magnification, ×3900 (bar represents 500 nm). (C) Spinning-disk confocal fluorescence microscopy images of a MEG-01 cell fixed and immunostained with LAMP2 and MRP4 antibodies to label DGs (MOC = 0.63 ± 0.06, n = 5 cells). Bar represents 10 μm. (D) Spinning-disk confocal fluorescence microscopy images of a live MEG-01 cell expressing the DG markers VMAT2-Cherry and LAMP2-GFP (MOC = 0.58 ± 0.03, n = 10 cells). Bar represents 5 μm. PM indicates plasma membrane; and IDG, immature dense granule.
MEG-01 cells have DGs that can be studied by different microscopy techniques. (A) Model depicting the protein traffic to DG. DG transmembrane proteins (gold) follow the secretory pathway through the Golgi complex to early endosomes where they are selectively targeted to maturing DGs originated from MVBs. (B) Thin-section transmission electron microscopy image of a MEG-01 cell subjected to HPF, fixed or embedded with glutaraldehyde-uranyl acetate-Lowicryl HM20. Original magnification, ×3900 (bar represents 500 nm). (C) Spinning-disk confocal fluorescence microscopy images of a MEG-01 cell fixed and immunostained with LAMP2 and MRP4 antibodies to label DGs (MOC = 0.63 ± 0.06, n = 5 cells). Bar represents 10 μm. (D) Spinning-disk confocal fluorescence microscopy images of a live MEG-01 cell expressing the DG markers VMAT2-Cherry and LAMP2-GFP (MOC = 0.58 ± 0.03, n = 10 cells). Bar represents 5 μm. PM indicates plasma membrane; and IDG, immature dense granule.
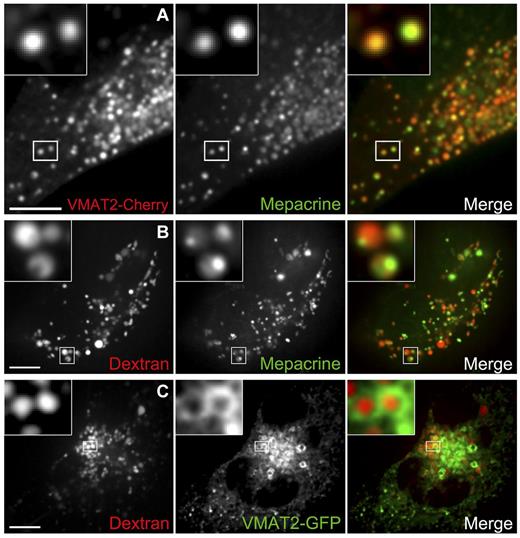
DGs originate from late endocytic structures. (A) DGs were labeled with the green fluorescent dye mepacrine in live MEG-01 cells expressing VMAT2-Cherry and visualized by spinning-disk confocal microscopy. The inset shows examples of colocalization between the 2 DG markers; 94% ± 2% of 171 mepacrine structures (7 cells) also contain VMAT-Cherry. (B) Live MEG-01 cells were allowed to internalize the fluid phase marker dextran Alexa Fluor 647, and DGs were subsequently labeled with mepacrine. Cells were observed by spinning-disk confocal fluorescence microscopy. Examples of structures containing both markers are presented in the magnified inset (MOC = 0.43 ± 0.04, n = 6 cells). (C) Live MEG-01 cells expressing the DG marker VMAT2-GFP were labeled with dextran Alexa Fluor 647 and imaged by spinning-disk confocal fluorescence microscopy. Inset: Examples of VMAT-GFP presence in the limiting membrane of organelles containing dextran Alexa Fluor 647 in the lumen; 66% ± 8% of 187 structures containing fluorescent dextran (7 cells) also contain VMAT-GFP. Bars represent 5 μm.
DGs originate from late endocytic structures. (A) DGs were labeled with the green fluorescent dye mepacrine in live MEG-01 cells expressing VMAT2-Cherry and visualized by spinning-disk confocal microscopy. The inset shows examples of colocalization between the 2 DG markers; 94% ± 2% of 171 mepacrine structures (7 cells) also contain VMAT-Cherry. (B) Live MEG-01 cells were allowed to internalize the fluid phase marker dextran Alexa Fluor 647, and DGs were subsequently labeled with mepacrine. Cells were observed by spinning-disk confocal fluorescence microscopy. Examples of structures containing both markers are presented in the magnified inset (MOC = 0.43 ± 0.04, n = 6 cells). (C) Live MEG-01 cells expressing the DG marker VMAT2-GFP were labeled with dextran Alexa Fluor 647 and imaged by spinning-disk confocal fluorescence microscopy. Inset: Examples of VMAT-GFP presence in the limiting membrane of organelles containing dextran Alexa Fluor 647 in the lumen; 66% ± 8% of 187 structures containing fluorescent dextran (7 cells) also contain VMAT-GFP. Bars represent 5 μm.
DGs have a late endocityc origin
It has been proposed that DGs,8 similar to AGs,31 originate from MVBs. To test this idea, we used a fluid phase marker taken up by endocytosis, fluorescent dextran, to label late endocytic structures. In nonspecialized cell types, this marker remains in the lumen of endocytic organelles through their progress from early/recycling endosomes to MVBs/late endosomes and it finally accumulates in lysosomes, the terminal organelle of the endocytic pathway. Pulse-chase experiments in MEG-01 cells show fluorescent dextran follows a similar early/recycling endosome to MVBs/late endosomes path in these specialized cells (supplemental Figure 6). We reasoned that if DGs originate from MVBs, internalized fluorescent dextran should accumulate in DGs in megakaryocytic cells (Figure 1A). We allowed MEG-01 cells to internalize fluorescent dextran, subsequently labeled DGs with mepacrine, and analyzed them by live cell fluorescence microscopy (Figure 2B). The colocalization of dextran and mepacrine (MOC = 0.43 ± 0.04) supports the idea of DGs originating from MVBs. Interestingly, although dextran labels the lumen of the DG in a rather uniform manner, mepacrine often seems more concentrated in an internal region of the DG, perhaps labeling the dense core (Figure 2B inset and supplemental Video 2). In a separate series of experiments, MEG-01 cells expressing VMAT2-GFP were labeled with internalized fluorescent dextran (Figure 2C). VMAT2-GFP shows green “doughnut”-shaped structures—the membrane of DGs imaged at their median plane—filled with red dextran (66% ± 8% of the red dextran structures contain both markers), consistent with an MVB origin of DGs. Similar results were obtained with LAMP2-GFP and internalized fluorescent dextran (supplemental Figure 4C).
Normal transport of membrane proteins to the DG depends on sorting signals bound by AP-3
DG integral membrane proteins may depend on tyrosine-based sorting signals, dileucine-based sorting signals, or both, in their cytosolic tails for vesicular transport to the organelle. Both LAMP2 and VMAT2 have sequences conforming to the tyrosine- and dileucine-based signal consensus, respectively. We subjected the putative signals to site-directed mutagenesis and analyzed the cellular localization of the mutant proteins compared with the wild-type proteins. Both LAMP2 and VMAT2 signal mutants were mistargeted to the plasma membrane, indicating a severe transport defect (Figure 3A-D). This result indicates transport to DGs is mediated by similar sorting signals as transport to melanosomes and lysosomes that are recognized by AP-3. These results are consistent with a model in which AP-3 is a key adaptor that packages proteins in vesicles at early endosome–associated tubules for subsequent transport to the maturing DGs (Figure 1A). Mutant cargo is unable to bind to AP-3, accumulates in early endosomal membranes, and leaks into the recycling pathway to the plasma membrane (Figures 1A, 3A-D, and 7J). An alternative explanation would be that LAMP2 and VMAT2 normally traffic through the plasma membrane on their way to the DG in an AP-3–independent pathway, but their mutant forms fail to be internalized by the endocytic adaptor AP-2. To test that possibility, wild-type LAMP2 and VMAT2 were expressed in MEG-01 cells subjected to AP-2 knockdown. Neither cargo was observed at the plasma membrane (data not shown), arguing against biosynthetic traffic through the plasma membrane en route to DGs, thus supporting the AP-3 model.
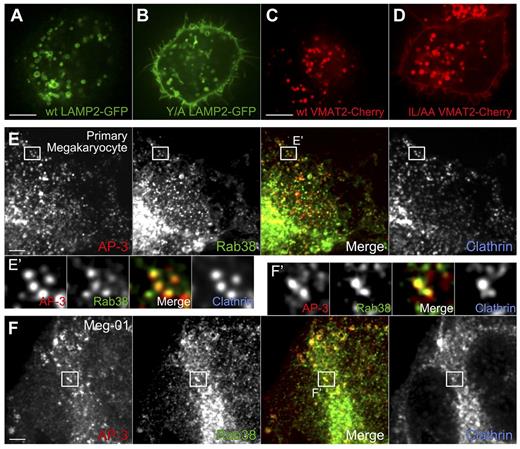
Sorting signals bound by AP-3 are crucial for the correct targeting of DG proteins in MEG-01 cells. AP-3 partially colocalizes with Rab38 in MKs and MEG-01 cells. (A-B) A single mutation in the LAMP2 cytosolic tail sorting signal (Y/A LAMP2: YEQF into AEQF) is sufficient to mistarget Y/A LAMP2-GFP to the plasma membrane in MEG-01 cells. Live cells were visualized by spinning-disk confocal fluorescence microscopy. (C-D) Similarly, mutation of the VMAT2 cytosolic tail sorting signal (IL/AA VMAT2: EEKMAIL into EEKMAAA) causes mistrafficking of the mutant protein to the plasma membrane in MEG-01 cells. Live cells were visualized by spinning-disk confocal fluorescence microscopy. (E-E′) Primary MKs were fixed and immunostained with antibodies against AP-3, Rab38, and clathrin, and imaged by spinning-disk confocal fluorescence microscopy. (E′) Close-up view of individual structures allows observation of colocalization of AP-3 and Rab38 (merge panel, MOC = 0.34 ± 0.01, n = 7 cells), whereas clathrin is also present in many of these structures (Rab38 and clathrin MOC = 0.33 ± 0.02, n = 7 cells). (F-F′) MEG-01 cells were fixed and immunostained with antibodies against AP-3, Rab38, and clathrin and imaged by spinning-disk confocal fluorescence microscopy. (F′) Similarly to the results obtained with MKs, AP-3 and Rab38 colocalize in structures that in many cases contain clathrin (Rab38 and AP-3 MOC = 0.32 ± 0.02, n = 4 cells; Rab38 and clathrin MOC = 0.33 ± 0.03, n = 4 cells). Bars represent 5 μm.
Sorting signals bound by AP-3 are crucial for the correct targeting of DG proteins in MEG-01 cells. AP-3 partially colocalizes with Rab38 in MKs and MEG-01 cells. (A-B) A single mutation in the LAMP2 cytosolic tail sorting signal (Y/A LAMP2: YEQF into AEQF) is sufficient to mistarget Y/A LAMP2-GFP to the plasma membrane in MEG-01 cells. Live cells were visualized by spinning-disk confocal fluorescence microscopy. (C-D) Similarly, mutation of the VMAT2 cytosolic tail sorting signal (IL/AA VMAT2: EEKMAIL into EEKMAAA) causes mistrafficking of the mutant protein to the plasma membrane in MEG-01 cells. Live cells were visualized by spinning-disk confocal fluorescence microscopy. (E-E′) Primary MKs were fixed and immunostained with antibodies against AP-3, Rab38, and clathrin, and imaged by spinning-disk confocal fluorescence microscopy. (E′) Close-up view of individual structures allows observation of colocalization of AP-3 and Rab38 (merge panel, MOC = 0.34 ± 0.01, n = 7 cells), whereas clathrin is also present in many of these structures (Rab38 and clathrin MOC = 0.33 ± 0.02, n = 7 cells). (F-F′) MEG-01 cells were fixed and immunostained with antibodies against AP-3, Rab38, and clathrin and imaged by spinning-disk confocal fluorescence microscopy. (F′) Similarly to the results obtained with MKs, AP-3 and Rab38 colocalize in structures that in many cases contain clathrin (Rab38 and AP-3 MOC = 0.32 ± 0.02, n = 4 cells; Rab38 and clathrin MOC = 0.33 ± 0.03, n = 4 cells). Bars represent 5 μm.
Rab32 and Rab38 partially colocalize with AP-3 in MKs and MEG-01 cells
Rab32 and Rab38 may cooperate with AP-3 in a pathway to deliver transmembrane proteins to DGs. We tested for colocalization of these proteins in both MEG-01 cells and primary MKs. Cells were fixed or permeabilized, and simultaneously immunostained with antibodies to Rab38, AP-3, and the vesicle coat protein clathrin. As shown in Figure 3E and F, Rab38 partially colocalizes with AP-3 in both MEG-01 cells and primary MKs (MOC = 0.32 ± 0.04 and 0.34 ± 0.03, respectively). This result indicates Rab38 is present at exit sites in early endosome–associated tubules, transport vesicles that have already pinched off and are en route to fuse with maturing DGs, or both. The presence of clathrin in many of the Rab38/AP-3 structures (MOC = 0.33 ± 0.05 and 0.33 ± 0.04 for MEG-01 and MKs, respectively) is consistent with Rab38 being recruited during vesicle budding or soon after it pinched off from the donor organelle. The fact that a similar result was obtained with MKs and MEG-01 cells further supports this cell line as an appropriate model system for studying DG biogenesis. Similar results were obtained for Rab32-stained primary MKs and MEG-01 cells (supplemental Figure 7). These data are consistent with a role of Rab32 in the biogenesis of DGs similar to that of Rab38.
Rab32 and Rab38 are predominantly present in immature DGs
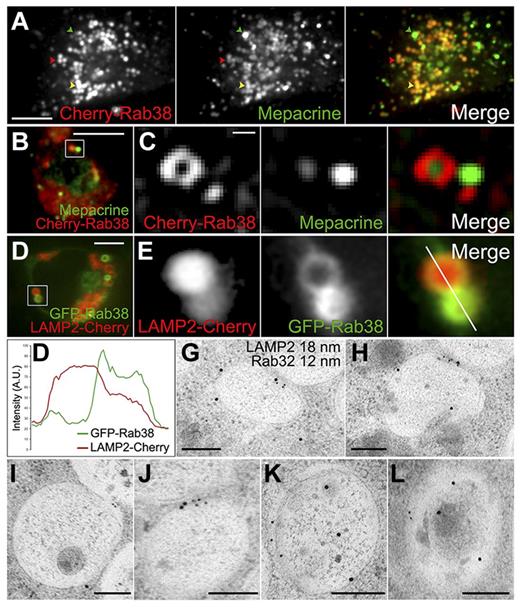
To test for the presence of Rab32 and Rab38 in DGs, we determined the localization of both Cherry-Rab38 and Cherry-Rab32 in live MEG-01 cells labeled with mepacrine. As shown in Figure 4A, Cherry-Rab38 presents a high degree of localization to mepacrine labeled structures. Interestingly, although some structures contain intermediate levels of both Cherry-Rab38 and mepacrine, others show the highest amount of Cherry-Rab38 and low amounts of mepacrine and vice versa (see supplemental Figure 8B for quantification). Similar results were obtained using Cherry-Rab32 (supplemental Figure 8). Mepacrine accumulates in DG and mature DGs display maximal mepacrine fluorescence; immature granules stain less strongly.32 Thus, the inverse correlation observed between the fluorescence intensity of each Rab versus mepacrine indicates the Rabs are enriched in immature DGs. A close look at MEG-01 cells expressing Cherry-Rab38 also reveals small structures (100-200 nm in diffraction limited puncta) that do not contain mepacrine and likely represent transport vesicles (Figure 4C and supplemental Video 3).
Rab32 and Rab38 are primarily present in immature DGs. (A-C) DGs were labeled with mepacrine in live MEG-01 cells expressing Cherry-Rab38 and visualized by spinning-disk confocal fluorescence microscopy; 95% ± 2% of structures containing Cherry-Rab38 (40 cells) also contain mepacrine. (A) A structure containing the highest amount of mepacrine and low Cherry-Rab38 levels is indicated with a green arrowhead, a structure with high concentration of Cherry-Rab38 and low mepacrine with a red arrowhead, and a structure with intermediate amounts of both markers with a yellow arrowhead (bar represents 5 μm). (B) Both organelles and vesicles are labeled with Cherry-Rab38, which is shown as an inset in panel C (bar represents 5 μm). (C) Although mepacrine is only present in the organelles, Cherry-Rab38 is also present in the vesicle (bar represents 500 nm). (D) Live MEG-01 cell coexpressing LAMP2-Cherry, as a DG marker, and GFP-Rab38 imaged by spinning-disk confocal fluorescence microscopy; 88 ± 4% of structures containing GFP-Rab38 (37 cells) also contain LAMP2-Cherry. Bar represents 5 μm. (E) A close-up view of structures from panel D shows a reverse correlation between the amount of Rab38 and the DG marker. (F) Fluorescence intensity line scan of the structures shown in panel E (merge panel). A.U., arbitrary units. (G-L) Immunogold electron microscopy images of immature DGs from MEG-01 cells subjected to HPF using antibodies against LAMP2 (18 nm) and Rab32 (12 nm). LAMP2 is present in 73% of the organelles label with Rab32 (n = 84 organelles). Original magnifications were ×15,000, ×20,000, ×15,000, ×20,000, ×25,000, and ×20,000, respectively. Bars represent 200 nm.
Rab32 and Rab38 are primarily present in immature DGs. (A-C) DGs were labeled with mepacrine in live MEG-01 cells expressing Cherry-Rab38 and visualized by spinning-disk confocal fluorescence microscopy; 95% ± 2% of structures containing Cherry-Rab38 (40 cells) also contain mepacrine. (A) A structure containing the highest amount of mepacrine and low Cherry-Rab38 levels is indicated with a green arrowhead, a structure with high concentration of Cherry-Rab38 and low mepacrine with a red arrowhead, and a structure with intermediate amounts of both markers with a yellow arrowhead (bar represents 5 μm). (B) Both organelles and vesicles are labeled with Cherry-Rab38, which is shown as an inset in panel C (bar represents 5 μm). (C) Although mepacrine is only present in the organelles, Cherry-Rab38 is also present in the vesicle (bar represents 500 nm). (D) Live MEG-01 cell coexpressing LAMP2-Cherry, as a DG marker, and GFP-Rab38 imaged by spinning-disk confocal fluorescence microscopy; 88 ± 4% of structures containing GFP-Rab38 (37 cells) also contain LAMP2-Cherry. Bar represents 5 μm. (E) A close-up view of structures from panel D shows a reverse correlation between the amount of Rab38 and the DG marker. (F) Fluorescence intensity line scan of the structures shown in panel E (merge panel). A.U., arbitrary units. (G-L) Immunogold electron microscopy images of immature DGs from MEG-01 cells subjected to HPF using antibodies against LAMP2 (18 nm) and Rab32 (12 nm). LAMP2 is present in 73% of the organelles label with Rab32 (n = 84 organelles). Original magnifications were ×15,000, ×20,000, ×15,000, ×20,000, ×25,000, and ×20,000, respectively. Bars represent 200 nm.
To corroborate the presence of Rab38 in DGs, particularly in immature organelles, we investigated the localization of GFP-Rab38 relative to LAMP2-Cherry as a DG marker. Again, we detected significant colocalization and structures that show the highest amount of the DG marker contain less GFP-Rab38 and vice versa (Figure 4D-F; see supplemental Figure 8B for quantification). Similarly, a reverse correlation was observed for the labeling intensity of Rab32 and LAMP2 in MEG-01 cells (supplemental Figure 8C), suggesting that both Rabs are present primarily in immature DGs.
Thin-section immunogold electron microscopy of MEG-01 cells stained for endogenous LAMP2 and Rab32 revealed the presence of both proteins in the same organelle, many of them immature DGs based on their low amount of internal dense material (Figure 4G-L). Specifically, 73% of the organelles labeled with Rab32 also contain LAMP2. Rab32 is restricted to the limiting membrane of the organelle, whereas LAMP2 was found both in the limiting membrane (Figure 4G,I,J) and inside of the organelle (Figure 4H,K,L). In addition, we observed LAMP2 associated with internal vesicles of MVBs and immature DGs (supplemental Figure 9).
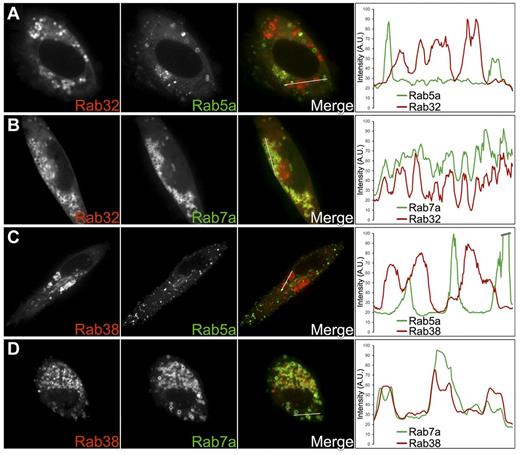
Rab5 is a well-known marker for early/recycling endosomes and Rab7 for MVBs/late endosomes. Rab7 also has been found in LROs such as melanosomes and lamellar bodies.33,34 We reasoned that if MVBs/late endosomes are DG precursors, and Rab32 and Rab38 localize primarily to immature DGs, these Rabs should colocalize preferentially with Rab7 but only marginally with Rab5. Indeed, confocal fluorescence microscopy images and corresponding fluorescence intensity line scans showed significant colocalization between GFP-Rab7a and either Cherry-Rab32 or Cherry-Rab38 (MOC = 0.52 ± 0.02 and 0.42 ± 0.03, respectively; Figure 5B,D). In contrast, colocalization between GFP-Rab5a and either Cherry-Rab32 or Cherry-Rab38 was minimal (MOC = 0.16 ± 0.02 and 0.14 ± 0.01, respectively; Figure 5A,C). These results indicate Rab32 and Rab38 localize primarily to immature DGs and that these organelles are closely related to MVBs/late endosomes, consistent with the dextran internalization results.
Rab32 and Rab38 colocalize with the late endocytic marker Rab7a but not with the vacuolar early endosome and recycling endosome marker Rab5a. Cherry-Rab32 and Cherry-Rab38 were cotransfected with GFP-Rab5a or GFP-Rab7a in MEG-01 cells. Confocal fluorescence microscopy images of live cells together with the corresponding fluorescence intensity line scan graphs are shown for each experiment. The white lines in the merge panels indicate the portions of the cells where fluorescence intensities for both the red and green channels were measured. (A,C) Neither Rab32 nor Rab38 colocalizes significantly with Rab5a, a marker of recycling vesicles/early endosomes (MOC = 0.16 ± 0.02, n = 13 cells and MOC = 0.14 ± 0.01, n = 7 cells, respectively). (B,D) Consistent with Rab32 and Rab38 being present in immature DGs, both proteins colocalize with Rab7a, a marker of late endosomal compartments that is also present in other LROs such as melanosomes and lamellar bodies (MOC = 0.52 ± 0.02, n = 7 cells and MOC = 0.42 ± 0.03, n = 7 cells, respectively).
Rab32 and Rab38 colocalize with the late endocytic marker Rab7a but not with the vacuolar early endosome and recycling endosome marker Rab5a. Cherry-Rab32 and Cherry-Rab38 were cotransfected with GFP-Rab5a or GFP-Rab7a in MEG-01 cells. Confocal fluorescence microscopy images of live cells together with the corresponding fluorescence intensity line scan graphs are shown for each experiment. The white lines in the merge panels indicate the portions of the cells where fluorescence intensities for both the red and green channels were measured. (A,C) Neither Rab32 nor Rab38 colocalizes significantly with Rab5a, a marker of recycling vesicles/early endosomes (MOC = 0.16 ± 0.02, n = 13 cells and MOC = 0.14 ± 0.01, n = 7 cells, respectively). (B,D) Consistent with Rab32 and Rab38 being present in immature DGs, both proteins colocalize with Rab7a, a marker of late endosomal compartments that is also present in other LROs such as melanosomes and lamellar bodies (MOC = 0.52 ± 0.02, n = 7 cells and MOC = 0.42 ± 0.03, n = 7 cells, respectively).
DGs can be studied biochemically in MEG-01 cells
To complement our microscopy studies, we carried out a subcellular fractionation of MEG-01 cells. A postnuclear supernatant obtained from a MEG-01 total extract was subjected to a 10% to 60% linear sucrose gradient. Figure 6A shows an immunoblotting analysis of each of the 11 fractions collected (Fs), with F1 being the least dense. Markers for different cell compartments are as follow: MRP4 and LAMP2 for immature and mature DGs, platelet factor-4 (PF-4) for AGs35 ; and Rab7a and Rab32 for cytosol, vesicles, and immature DGs. The DG markers LAMP2 and MRP4 coelute in F6 to F9, suggesting these fractions contain an heterogeneous mixture of DGs at different levels of maturation. LAMP2 is also present as a faint band in F1 that possibly corresponds to the vesicular pool of LAMP2. AGs eluted in F4 as detected by PF-4, consistent with their lower density compared with DGs, and reproducing the findings of other groups with platelet organelles.29 Rab32 and Rab7 presence in F1 and F2 corresponds to their cytosolic and vesicle-associated pools. Interestingly, Rab32 and Rab7 also coelute with the DGs markers in F6 to F8, suggesting these fractions contain immature DGs.
Biochemical study of MEG-01 dense granules. (A) Immunoblotting analysis of fractions obtained from MEG-01 postnuclear supernatants subjected to subcellular fractionation with a 10% to 60% sucrose gradient. Markers for different cell compartments are as follow: MRP4 and LAMP2 for immature and mature DGs; PF-4 for α granules; and Rab7a and Rab32 for cytosol, vesicles, and DGs. (B) ADP (μM, solid lines) and fluorescence intensity of both infrared fluorescent-dextran (A.U., dashed line) and the fluorescent Ca2+ indicator Oregon Green BAPTA-1 dextran (A.U., dotted line) in fractions from panel A. The concentration of ADP was determined both in the untreated sucrose gradient fractions (solid circles) and in sucrose gradient fractions enriched in MRP4 structures by immunoprecipitation using an MRP4 antibody (open circles). (C) Sucrose gradient fractions were immunoprecipitated using a Rab38 antibody and the presence of MRP4 in the precipitated structures was determined by immunoblotting, confirming the occurrence of the proteins in the same structures. (D) The coexistence of both LAMP2 and MRP4 in the same organelles was confirmed by coimmnunoprecipitation using a MRP4 antibody (left) or a LAMP2 antibody (right) and immunoblotting analysis using an antibody against the other protein. SGF indicates sucrose gradient fraction; IP, immunoprecipitation; IB, immunoblotting; and IDG, immature DG.
Biochemical study of MEG-01 dense granules. (A) Immunoblotting analysis of fractions obtained from MEG-01 postnuclear supernatants subjected to subcellular fractionation with a 10% to 60% sucrose gradient. Markers for different cell compartments are as follow: MRP4 and LAMP2 for immature and mature DGs; PF-4 for α granules; and Rab7a and Rab32 for cytosol, vesicles, and DGs. (B) ADP (μM, solid lines) and fluorescence intensity of both infrared fluorescent-dextran (A.U., dashed line) and the fluorescent Ca2+ indicator Oregon Green BAPTA-1 dextran (A.U., dotted line) in fractions from panel A. The concentration of ADP was determined both in the untreated sucrose gradient fractions (solid circles) and in sucrose gradient fractions enriched in MRP4 structures by immunoprecipitation using an MRP4 antibody (open circles). (C) Sucrose gradient fractions were immunoprecipitated using a Rab38 antibody and the presence of MRP4 in the precipitated structures was determined by immunoblotting, confirming the occurrence of the proteins in the same structures. (D) The coexistence of both LAMP2 and MRP4 in the same organelles was confirmed by coimmnunoprecipitation using a MRP4 antibody (left) or a LAMP2 antibody (right) and immunoblotting analysis using an antibody against the other protein. SGF indicates sucrose gradient fraction; IP, immunoprecipitation; IB, immunoblotting; and IDG, immature DG.
To identify the sucrose gradient fraction(s) that contained the most mature DGs, the levels of ADP were determined for each fraction (Figure 6B filled circles). A high amount of ADP was measured in F1 to F3 that suggests the cytosolic pool of ADP (F1) leaked into F2 and F3. Importantly, F9, which contains the DG markers LAMP2 and MRP4, presented a small but reproducible ADP peak. MRP4 has been suggested to be the ADP transporter of the DG,28 so we enriched the sucrose gradient fractions in MRP4-containing structures by immunoprecipitation using an anti-MRP4 antibody. After immunoprecipitation, F9 showed an increase of ∼ 5 times in the amount of ADP, which coincided with the concentration factor of the sample because of immunoprecipitation (Figure 6B open circles). This result indicates that F9 contains the most mature DGs and that immature DGs present in F6 to F8 are not competent for ADP accumulation. The larger amount of MRP4 and LAMP2 in fractions F6 to F8 containing immature DGs compared with F9 representing mature DGs is also consistent with the electron microscopy data showing more MVBs and immature DGs than mature DGs in MEG-01 cells (Figure 1B).
The ability to fractionate the organelles biochemically provided another angle to test the idea that DGs originate from MVBs. For this purpose, MEG-01 cells were allowed to internalize fluorescent dextran and a cellular extract fractionated in a sucrose gradient. The fluorescence intensity of each fraction was measured and the results presented in Figure 6B (dashed line). F9 shows the highest peak, indicating DGs contain internalized fluid phase material, thus supporting the idea of DGs originating from MVBs. The presence of dextran in F4, the fraction containing the AGs labeled with PF-4, likely reflects their published MVB origin.31 Taking advantage of the high Ca2+ content of dense granules, we used the fluorescent Oregon Green 488 BAPTA-1 calcium indicator conjugated to dextran. MEG-01 cells were allowed to internalize this compound, which increases its fluorescence intensity 14-fold upon Ca2+ binding. After density gradient fractionation, the fluorescence intensity of each fraction was measured and the results presented in Figure 6B (dotted line). The peak in F9 displays most of the fluorescence indicating this fraction contains mature, Ca2+-containing DGs.
We found that in MEG-01 cells the DG marker LAMP2 colocalizes with Rab32 and Rab38 by confocal fluorescence microscopy of overexpressed proteins and with Rab32 by electron microscopy of endogenous proteins. To support those results with biochemical data, sucrose gradient fractions were immunoprecipitated using an anti-Rab38 antibody. As shown in Figure 6C, structures labeled by Rab38 also contain MRP4, predominantly in F6 to F8, which most likely correspond to immature DGs. Although very faint, MRP4 bands in F2 and F3 suggest Rab38 and MRP4 coexist in transport vesicles.
Finally, immunoprecipitation experiments were carried out to verify that LAMP2 and MRP4 not only coelute in the same fractions but that they are present in the same organelles. F8 and F9 were submitted to immunoprecipitation using either anti-MRP4 or anti-LAMP2 antibodies. Immunoblotting analysis of the eluted proteins indicated MRP4 is able to pull down structures containing LAMP2 and vice versa (Figure 6D), indicating they are present in the same organelles in MEG-01 cells as previously described for platelets DGs.28,29 A control experiment using an irrelevant antibody demonstrates the specificity of the immunoprecipitation procedure (supplemental Figure 10A). Immunoblotting for PF-4 shows AGs are not being nonspecifically isolated along with DGs (supplemental Figure 10B).
Rab32 and Rab38 are involved either in tethering or fusion of cargo-containing vesicles with the immature DG, or both
To further investigate the function of Rab32 and Rab38 in DG biogenesis, MEG-01 cells were subjected to siRNA knockdown of either Rab32 or Rab38 and expression of LAMP2-Cherry as a DG protein cargo reporter. In control cells, LAMP2-Cherry labels organelle-sized structures by live cell imaging (Figure 7A). In contrast, in Rab32-deficient cells LAMP2-Cherry is present in structures significantly smaller and more consistent with vesicles or very small organelles (Figure 7C,G). Rab38-deficient cells show a similar phenotype but to a lesser extent (Figure 7E,G). What is more, in Rab-deficient cells most of the LAMP2 structures move significantly faster than in the control cells, which is also consistent with LAMP2 being present in vesicles (Figure 7H; also, compare kymographs in Figure 7B with D and F and supplemental Videos 4-6). These results suggest Rab32 and Rab38 are crucial components in either tethering or fusion events (or both) between the cargo-carrying vesicle and the maturing organelle. In addition, the fact that deficiency of either Rab cannot be completely compensated by the presence of the other one indicates they are not fully redundant at least for this function.
Rab32 or Rab38 knock-down impairs normal fusion of vesicles containing dense granule proteins with the organelle. (A-F) MEG-01 cells were cotransfected with LAMP2-Cherry as a DG reporter and either control siRNA shown in panels A and B, Rab32 siRNA in panels C and D, or Rab38 siRNA in panels E and F (see supplemental Figure 11 for Rab32/38 siRNA knockdown confirmation by immunoblotting). The kymographs presented in panels B, D, and F were made by aligning on a time axis the pieces of images indicated with a white rectangle in panels A, C, and E, respectively, from each of the 60 frames of the corresponding movies (1 frame/second). (A) Control siRNA cells present diffraction-limited LAMP2 structures consistent in size with organelles. (B) The LAMP2 structures in Control siRNA cells present a limited range of motion. (C,E) Both Rab32 and Rab38 siRNA cells present LAMP2 structures that are more consistent in size with vesicles or small organelles. (D,F) The smaller LAMP2 structures in Rab32 and Rab38 siRNA cells are more dynamic and move faster than structures in Control siRNA cells. (G) The diameter of LAMP2 structures present in the representative cells shown in panels A, C, and E was measured using the Ruler function in Slidebook. (H) The average speed of LAMP2 structures present in the representative cells shown in panels A, C, and E was measured using the Manual Particle Tracking function in Slidebook. (I) Extracts from control, Rab32, and Rab38 siRNA-treated cells were fractionated in sucrose gradients. For each treatment, the amount of LAMP2 in the first fraction of the gradient, which corresponds to the vesicular LAMP2, was analyzed by immunoblotting. The levels of tubulin in the same blot were used to confirm equal loading. Bars represent 5 μm (*P < .05; **P < .001). (J) DG membrane proteins are sorted in early endosomal compartments by adaptor protein complexes, such as AP-3, which recognize sorting signals present in their cytosolic tails. Rab32 and Rab38 are recruited to the nascent clathrin-coated vesicle and through interactions with so far unknown effectors target the vesicle to the maturing DG. The DG precursor is a MVB that on receiving Rab32/Rab38 vesicles containing DG proteins, such as the ADP transporter MRP4 or the serotonin transporter VMAT2, matures into a DG.
Rab32 or Rab38 knock-down impairs normal fusion of vesicles containing dense granule proteins with the organelle. (A-F) MEG-01 cells were cotransfected with LAMP2-Cherry as a DG reporter and either control siRNA shown in panels A and B, Rab32 siRNA in panels C and D, or Rab38 siRNA in panels E and F (see supplemental Figure 11 for Rab32/38 siRNA knockdown confirmation by immunoblotting). The kymographs presented in panels B, D, and F were made by aligning on a time axis the pieces of images indicated with a white rectangle in panels A, C, and E, respectively, from each of the 60 frames of the corresponding movies (1 frame/second). (A) Control siRNA cells present diffraction-limited LAMP2 structures consistent in size with organelles. (B) The LAMP2 structures in Control siRNA cells present a limited range of motion. (C,E) Both Rab32 and Rab38 siRNA cells present LAMP2 structures that are more consistent in size with vesicles or small organelles. (D,F) The smaller LAMP2 structures in Rab32 and Rab38 siRNA cells are more dynamic and move faster than structures in Control siRNA cells. (G) The diameter of LAMP2 structures present in the representative cells shown in panels A, C, and E was measured using the Ruler function in Slidebook. (H) The average speed of LAMP2 structures present in the representative cells shown in panels A, C, and E was measured using the Manual Particle Tracking function in Slidebook. (I) Extracts from control, Rab32, and Rab38 siRNA-treated cells were fractionated in sucrose gradients. For each treatment, the amount of LAMP2 in the first fraction of the gradient, which corresponds to the vesicular LAMP2, was analyzed by immunoblotting. The levels of tubulin in the same blot were used to confirm equal loading. Bars represent 5 μm (*P < .05; **P < .001). (J) DG membrane proteins are sorted in early endosomal compartments by adaptor protein complexes, such as AP-3, which recognize sorting signals present in their cytosolic tails. Rab32 and Rab38 are recruited to the nascent clathrin-coated vesicle and through interactions with so far unknown effectors target the vesicle to the maturing DG. The DG precursor is a MVB that on receiving Rab32/Rab38 vesicles containing DG proteins, such as the ADP transporter MRP4 or the serotonin transporter VMAT2, matures into a DG.
Subcellular fractions were obtained from extracts of control, Rab32- and Rab38-deficient cells and the presence of endogenous LAMP2 in F1 was studied by immunoblotting (Figure 7I). The amount of LAMP2 in F1 from Rab32- and Rab38-deficient cells is strikingly higher than the control. This result indicates that in cells deficient for each Rab the amount of vesicular LAMP2 is higher than in control cells, confirming the data obtained with LAMP2-Cherry by fluorescence microscopy.
Discussion
The great level of difficulty in studying primary bone marrow MKs is a key reason behind our poor understanding of DG biogenesis. We demonstrate here that the megakaryocytic cell line MEG-01 is a very good model system to study DG biogenesis. The presence of mature DGs in MEG-01 cells was established by electron microscopy, mepacrine fluorescence microscopy in live cells, and ADP accumulation in MRP4-containing high-density organelles. Consistently, known DG protein markers such as endogenous LAMP2 and MRP4 or exogenously expressed LAMP2 and VMAT2 colocalize in MEG-01 cells, indicating the DG biogenesis pathways are mostly conserved. DG markers segregate from AG markers by both microscopy and biochemical fractionation. MEG-01 cells present a high proportion of MVBs and immature DGs, indicating these cells represent relatively immature megakaryocytes. Despite the similarities between dense granules in MEG-01 and primary MKs, potential limitations of using megakaryocytic leukemia cells should be kept in mind.
Unlike most secretory organelles, AGs and DGs may not originate from the trans-Golgi network. More than a decade ago, MVBs/late endosomes were shown to be the precursors of AGs.31 One study proposed a similar MVB origin for DGs on the basis of the localization of granulophysin, a marker later revealed as CD63/LAMP3, which is also present in AGs and various other cellular compartments.8,9 Our use of an internalized fluid phase marker to label late endocytic compartments together with well-validated DG markers (mepacrine, LAMP2, ADP) shows unequivocally that DGs originate from endosomal compartments rather than the trans-Golgi network. Consistently, the putative serotonin transporter (VMAT2) also colocalize with internalized dextran. Furthermore, the presence of the DG protein LAMP2 in the limiting membrane and internal vesicles of MVBs was revealed by immunogold electron microscopy. Rab7 is a classic marker of MVBs/late endosomes; therefore, its colocalization by microscopy and cofractionation in density gradients with Rab32, Rab38, and DG protein markers further supports a MVBs/late endosome origin for DGs.
In addition to endocytic material, DGs must receive newly synthesized integral membrane proteins that normally function in its limiting membrane. Our results show for the first time that tyrosine- and dileucine-based sorting signals are required for normal traffic to DGs of LAMP2 and VMAT2, respectively. These types of signals are also used for AP-3–mediated transport to melanosomes and lysosomes, suggesting the mechanism of biogenesis is highly conserved among different LROs. Our results are most consistent with a model in which AP-3 carries out transport of newly synthesized DG membrane proteins from early endosome–associated tubules to maturing DGs, a pathway analogous to that described for melanosomal proteins (Figure 7J).
Mutation of Rab38 causes deficiency of platelet DGs and other LROs in rodent models of HPS.15-17,34 Rab38 and its very close homolog Rab32 operate in a partially redundant manner in melanosome biogenesis.17,18 We found that Rab32 and Rab38 partially colocalize with AP-3 and clathrin both in primary MKs and MEG-01 cells. This result suggests Rab32 and Rab38 are recruited to cargo-filled vesicles budding from early endosome–associated tubules and likely destined for maturing DGs. Rab32 and Rab38 are also present predominantly in immature DGs (Figures 4,Figure 5–6 and supplemental Figure 8). We also detect small structures labeled by fluorescently tagged Rabs that likely represent transport vesicles. Overall, these localization experiments fit very well with a model in which Rab32 and Rab38 define a pathway from early endosome–associated tubules to maturing dense granules (Figure 7J). In this context, the LAMP2-Cherry trafficking phenotype observed on knock-down of either Rab32 or Rab38 are indicative of a deficiency in the tethering or fusion of vesicles containing DG cargo with the organelles. These results constitute the first evidence at the molecular level of Rab32 and Rab38 involvement in DGs biogenesis.
Our model for the biogenesis of DGs is depicted in Figure 7J. Newly synthesized DG proteins are sorted in specific early endodomal tubules by the recognition of sorting signals present in their cytosolic tails by adaptors such as AP-3. At this point or quickly after pinching off, cytosolic Rab32 and Rab38 are recruited to these vesicles and through interactions with still unknown effectors regulate docking and fusion of the vesicles with the MVBs/immature DGs.
This work represents an important step forward in our understanding of the molecular mechanism of platelet DG biogenesis. Moreover, our data indicate MEG-01 cells are a valid and powerful model system for the study of DG formation and the identification of new players involved in these pathways.
There is an Inside Blood commentary on this article in this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Andrew Peden for the AP-3 antibody and Thomas Giddings for HPF.
This work was supported by American Heart Association award 09SDG2280525 and National Institutes of Health grant 1R01HL106186-01A1 (S.M.D.). Microscopes used in this work are supported in part by the Colorado State University Microscope Imaging Network core infrastructure grant.
National Institutes of Health
Authorship
Contribution: A.L.A., J.A.B., and S.M.D. designed the study, performed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Santiago M. Di Pietro, Department of Biochemistry and Molecular Biology, 1870 Campus Delivery, Colorado State University, Fort Collins, CO 80523-1870; e-mail: santiago.dipietro@colostate.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal