Abstract
Dasatinib is effective therapy for newly diagnosed patients with chronic myeloid leukemia, but not all patients respond well. We analyzed the outcome of patients treated with dasatinib as first-line therapy to identify patients who are more likely to fare poorly. The 8.6% of patients who at 3 months had a BCR-ABL1/ABL1 ratio > 10% had a significantly worse 2-year cumulative incidence of complete cytogenetic response (58.8% vs 96.6%, P < .001) and molecular responses than the remaining patients with a lower transcript levels. The predictive value of the 3-month transcript level could be improved using the dasatinib-specific transcript level cut-offs, namely, 2.2%, 0.92%, and 0.57% for complete cytogenetic response, 3 log and 4.5 log reductions in the transcript level, respectively. The study was registered at www.clinicaltrials.gov as #NCT01460693.
Introduction
Second-generation tyrosine kinase inhibitors, such as dasatinib or nilotinib, have proved to be very successful in treating chronic myeloid leukemia (CML) patients who have failed imatinib.1,2 This observation led investigators to explore the efficacy of these drugs as first-line therapy in CML. Indeed, early reports with both of these second-generation tyrosine kinase inhibitors suggest a very high response rate3,4 ; but despite these encouraging results, some patients still fail to respond to front-line dasatinib or nilotinib and may benefit from an alternative treatment. In this study, we define molecular milestones at 3 months that may identify patients treated with dasatinib as first-line therapy who have a low probability of achieving an adequate response and are thus candidates for alternative treatment.
Methods
Patients and therapy
Between August 2008 and September 2011, 142 consecutive adult patients with CML in chronic phase received dasatinib 100 mg daily as first-line therapy as described elsewhere4 in the United Kingdom SPIRIT 2 study. Briefly, the United Kingdom SPIRIT 2 trial is a phase 3 study in which newly diagnosed CML patients in chronic phase are randomly allocated to receive either imatinib 400 mg daily or dasatinib 100 mg daily (www.clinicaltrials.gov; #NCT01460693). Patient characteristics are shown in Table 1. The median follow-up was 18.2 months (range, 12-35 months). Bone marrow morphology and cytogenetics were assessed at diagnosis and every 12 months thereafter. Chronic phase was defined using conventional criteria.5 Complete cytogenetic response (CCyR) was defined either by the failure to detect any Philadelphia chromosome-positive metaphases in bone marrow examinations with a minimum of 20 metaphases examined or by the reduction of the BCR-ABL1 transcript numbers in peripheral blood to a level usually regarded as consistent with CCyR (ie, 1%).6,7
Patient characteristics at diagnosis and relative risks for the achievement of CCyR, MR3, and MR4.5 in the univariate analysis
| Variable . | Value . | CCyR . | MR3 . | MR4.5 . |
|---|---|---|---|---|
| Sex, n (%) | P = .77 | P = .88 | P = .11 | |
| Male | 79 (55.6) | 1 | 1 | 1 |
| Female | 63 (44.4) | 0.948 | 1.030 | 1.564 |
| Age, y | P = .40 | P = .09 | P = .34 | |
| Median (range) | 54.4 (18-82) | 1.004 | 1.011 | 1.008 |
| Sokal risk group, n (%)* | P = .005 | P = .002 | P = .03 | |
| Low | 35 (29.9) | 1 | 1 | 1 |
| Intermediate | 51 (43.6) | 0.813 | 0.789 | 0.828 |
| High | 31 (26.5) | 0.510 | 0.350 | 0.328 |
| EUTOS risk group, n (%)† | P = .09 | P = .04 | P = .05 | |
| Low | 86 (83.5) | 1 | 1 | 1 |
| High | 17 (16.5) | 0.608 | 0.451 | 0.247 |
| Spleen size at diagnosis, cm | P = .11 | P = .02 | P = .13 | |
| Median (range) | 2 (0-32) | 0.976 | 0.953 | 0.958 |
| Hemoglobin level, g/dL | P < .001 | P < .001 | P < .001 | |
| Median (range) | 11.0 (4.2-15.8) | 1.225 | 1.267 | 1.367 |
| White blood cell count, × 109/L | P = .04 | P = .05 | P = .11 | |
| Median (range) | 56.3 (2-428) | 0.998 | 0.997 | 0.997 |
| Platelet count, × 109/L | P = .8 | P = .77 | P = .89 | |
| Median (range) | 425 (100-2433) | 1.000 | 1.000 | 1.000 |
| Blasts in peripheral blood, % | P = .045 | P = .09 | P = .29 | |
| Median (range) | 0.6 (0-14.5) | 0.898 | 0.895 | 0.895 |
| Basophils in peripheral blood, % | P = .54 | P = .74 | P = .13 | |
| Median (range) | 3.9 (0-19.0) | 0.989 | 0.993 | 0.946 |
| Variable . | Value . | CCyR . | MR3 . | MR4.5 . |
|---|---|---|---|---|
| Sex, n (%) | P = .77 | P = .88 | P = .11 | |
| Male | 79 (55.6) | 1 | 1 | 1 |
| Female | 63 (44.4) | 0.948 | 1.030 | 1.564 |
| Age, y | P = .40 | P = .09 | P = .34 | |
| Median (range) | 54.4 (18-82) | 1.004 | 1.011 | 1.008 |
| Sokal risk group, n (%)* | P = .005 | P = .002 | P = .03 | |
| Low | 35 (29.9) | 1 | 1 | 1 |
| Intermediate | 51 (43.6) | 0.813 | 0.789 | 0.828 |
| High | 31 (26.5) | 0.510 | 0.350 | 0.328 |
| EUTOS risk group, n (%)† | P = .09 | P = .04 | P = .05 | |
| Low | 86 (83.5) | 1 | 1 | 1 |
| High | 17 (16.5) | 0.608 | 0.451 | 0.247 |
| Spleen size at diagnosis, cm | P = .11 | P = .02 | P = .13 | |
| Median (range) | 2 (0-32) | 0.976 | 0.953 | 0.958 |
| Hemoglobin level, g/dL | P < .001 | P < .001 | P < .001 | |
| Median (range) | 11.0 (4.2-15.8) | 1.225 | 1.267 | 1.367 |
| White blood cell count, × 109/L | P = .04 | P = .05 | P = .11 | |
| Median (range) | 56.3 (2-428) | 0.998 | 0.997 | 0.997 |
| Platelet count, × 109/L | P = .8 | P = .77 | P = .89 | |
| Median (range) | 425 (100-2433) | 1.000 | 1.000 | 1.000 |
| Blasts in peripheral blood, % | P = .045 | P = .09 | P = .29 | |
| Median (range) | 0.6 (0-14.5) | 0.898 | 0.895 | 0.895 |
| Basophils in peripheral blood, % | P = .54 | P = .74 | P = .13 | |
| Median (range) | 3.9 (0-19.0) | 0.989 | 0.993 | 0.946 |
A total of 25 patients had missing data.
A total of 39 patients had missing data.
Detection of BCR-ABL1 transcripts
BCR-ABL1 transcripts were measured in the blood at 3-month intervals using quantitative RT-PCR as described previously.8,9 The tests were performed in the molecular biology laboratory at Hammersmith Hospital. Results were converted to the international scale.10 Molecular responses (MRs) were expressed according to the log reduction in the transcript level below the conventionally defined starting point of 100%. MR3 (equivalent to major molecular response) was defined as a transcript level ≤ 0.1% on the international scale. MR4.5 was defined as BCR-ABL1 ratio of 0.0032% on the international scale, provided copy numbers of the control were at least 45 000, consistent with a 4.5 log reduction in the transcript level. Complete molecular response (CMR) was defined by the finding of 2 consecutive samples with no detectable transcripts with an ABL1 control > 40 000 copies. Samples with an ABL1 control < 10 000 were discarded. For samples included in this study, the median copy number of the ABL1 control was 62 000.
Statistical methods
The probabilities of cytogenetic and molecular responses were calculated using the CI procedure, whereby cytogenetic or molecular responses were the events of interest and therapy discontinuation or death was the competitor. Probabilities of CI were examined using Fine-Gray regression.11-13 Univariate and multivariate analyses were performed in accordance with standard methods; variables found to be significant at the P < .10 level were entered in the multivariate analysis. Receiver operating characteristic curves were plotted using the SPSS Version 19 statistical package.
Results and discussion
Responses to dasatinib first-line therapy
The 2-year cumulative incidence of CCyR, MR3, MR4.5, and CMR was 89.8%, 70.0%, 39.1%, and 6.5%, respectively. We examined the influence of the patient characteristics shown in Table 1 on the probability of achieving cytogenetic and molecular responses. The only independent predictors for the achievement of CCyR were Sokal risk group (relative risk [RR] = 0.713 and RR = 0.582, P = .027 for intermediate and high Sokal, respectively) and the hemoglobin level at diagnosis (RR = 1.236, P < .001). Similarly, Sokal risk group (RR = 0.590 and RR = 0.412, P = .029 for intermediate and high Sokal, respectively) and the hemoglobin level (RR = 1.360, P < .001) were the only independent predictors for the achievement of MR3. Hemoglobin level at diagnosis was the only independent predictor for MR4.5.
The BCR-ABL1 transcript levels after 3 months on dasatinib therapy strongly predicts for cytogenetic and molecular responses
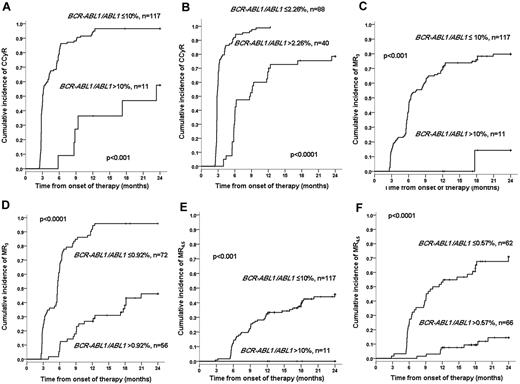
A total of 128 patients had a valid sample for quantitative RT-PCR for BCR-ABL1 at 3 months. In the remaining 14 patients, the sample was missing or had an ABL control < 10 000 copies. We examined the predictive value of the transcript level measured at 3 months on the probabilities of achieving cytogenetic and molecular responses. The 117 (91.4%) patients who after 3 months on dasatinib had a BCR-ABL1/ABL1 ratio of ≤ 10% had a significantly superior 2-year cumulative incidence of CCyR (91.4% vs 58.8%, P < .001), MR3 (79.8% vs 14.3%, P < .001), and MR4.5 (45.7% vs 0%, P < .001), compared with the 11 (8.6%) patients with values greater than 10% (Figure 1), but it was not predictive for CMR (6.1% vs 0%, P = .45). The 3-month transcript level (> 10%) and hemoglobin level at diagnosis were the only independent predictors for CCyR (RR = 0.239, P < .001 and RR = 1.248, P = .001, respectively) and MR4.5 (RR = 0.015, P < .001 and RR = 1.315, P = .01, respectively) The 3-month transcript level (RR = 0.067, P < .001), the hemoglobin at diagnosis (RR = 1.205, P = .002), and the Sokal risk group (low RR = 1; intermediate RR = 0.618 and high RR = 0.515; P = .04) were the only independent predictors for MR3.
Two-year cumulative incidence of CCyR, MR3, and CMR4.5 according to the 3-month BCR-ABL1 transcript level. (A-B) The cumulative incidence of CCyR according to whether the transcript level is > or < 10% (A) or 2.26% (B). (C-D) The cumulative incidence of MR3 according to whether the transcript level is > or < 10% (C) or 0.92% (D). (E-F) The cumulative incidence of MR4.5 according to whether the transcript level is > or < 10% (E) or 0.57% (F). The 2.26%, 0.92%, and 0.57% are the transcript level cut-offs identified using the receiver operating characteristic curves that optimally predict for each specific outcome.
Two-year cumulative incidence of CCyR, MR3, and CMR4.5 according to the 3-month BCR-ABL1 transcript level. (A-B) The cumulative incidence of CCyR according to whether the transcript level is > or < 10% (A) or 2.26% (B). (C-D) The cumulative incidence of MR3 according to whether the transcript level is > or < 10% (C) or 0.92% (D). (E-F) The cumulative incidence of MR4.5 according to whether the transcript level is > or < 10% (E) or 0.57% (F). The 2.26%, 0.92%, and 0.57% are the transcript level cut-offs identified using the receiver operating characteristic curves that optimally predict for each specific outcome.
The predictive power for cytogenetic and molecular responses of the BCR-ABL1 transcript level at 3 months can be greatly improved when therapy-specific transcript level cut-offs are used
As previously described,14 we identified cut-offs in the 3-month transcript levels that predicted for achievement of cytogenetic and molecular responses with the maximal sensitivity and specificity using a receiver operating characteristic curve. The optimal transcript level cut-offs identified for CCyR were 2.26% (2-year CI of CCyR 100% vs 78.5%, P < .001), for MR3 0.92% (2-year CI of MR3 95.8% vs 46.2%, P < .001), for MR4.5 0.57% (2-year CI of MR4.5 70.9% vs 14.5%, P < .001), and for CMR 0.24% (2-year CI of CMR 14.1% vs 1.8%, P = .004). When the multivariate analysis was repeated using these cut-offs, the BCR-ABL1 transcript level was the only independent predictor for CCyR, MR3, MR4.5, and CMR.
We have previously reported that patients treated with imatinib first-line therapy who have a 3-month transcript level > 9.84% have significantly worse survival and that the measurement of the transcript level at 3 months is the most accurate way to identify patients who will fare poorly.14 Similar results have been reported by others.15-18 The transcript level at 3 months (< 10%) can also be used to identify patients treated with second-line dasatinib or nilotinib who have a lower probability of survival.19 In this analysis, we show that the measurement of the transcript level at 3 months in patients treated with first-line dasatinib is also predictive for outcome, allowing the identification of approximately 10% of dasatinib-treated patients who have a low probability of achieving CCyR and deep molecular responses and for whom other forms of treatment might be considered. The predictive power of the 3-month quantitative RT-PCR assessment can be greatly improved by identifying and then applying the optimal transcript level cut-offs for each specific outcome (CCyR, MR3, and MR4.5). These cut-off values are significantly lower than the ones reported for imatinib (ie, 2% vs 10%), highlighting the fact that patients treated with second-generation tyrosine kinase inhibitors have response kinetics that differ from patients treated with imatinib and need specifically tailored therapeutic milestones.20
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institute for Health Research Biomedical Research Center Funding Scheme, United Kingdom.
Authorship
Contribution: D. Marin performed statistical analysis and wrote the manuscript; C.H. assembled the patient data and coordinated the study; R.E.C., J.A., D. Milojkovic, C.P., J.M.G., and S.O. conducted the clinical trial, provided patient care, and commented on the manuscript; and L.F. commented on the manuscript and performed the molecular studies.
Conflict-of-interest disclosure: D. Marin, J.A., D. Milojkovic, J.M.G., and S.O. received research support from Novartis and Bristol Myers-Squibb. R.E.C. received research funding and speaker fees from Bristol Myers-Squibb. The remaining authors declare no competing financial interests.
Correspondence: David Marin, Department of Haematology, Imperial College London, Du Cane Road, London W12 0NN, United Kingdom; e-mail: d.marin@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal