Abstract
Splenic sequestration of RBCs with reduced surface area and cellular deformability has long been recognized as contributing to pathogenesis of several RBC disorders, including hereditary spherocytosis. However, the quantitative relationship between the extent of surface area loss and splenic entrapment remains to be defined. To address this issue, in the present study, we perfused ex vivo normal human spleens with RBCs displaying various degrees of surface area loss and monitored the kinetics of their splenic retention. Treatment with increasing concentrations of lysophosphatidylcholine resulted in a dose-dependent reduction of RBC surface area at constant volume, increased osmotic fragility, and decreased deformability. The degree of splenic retention of treated RBCs increased with increasing surface area loss. RBCs with a > 18% average surface area loss (> 27% reduced surface area-to-volume ratio) were rapidly and completely entrapped in the spleen. Surface-deficient RBCs appeared to undergo volume loss after repeated passages through the spleen and escape from splenic retention. The results of the present study for the first time define the critical extent of surface area loss leading to splenic entrapment and identify an adaptive volume regulation mechanism that allows spherocytic RBCs to prolong their life span in circulation. These results have significant implications for understanding the clinical heterogeneity of RBC membrane disorders.
Introduction
During their 120-day life span, human RBCs repeatedly traverse capillaries of the vascular bed and interendothelial slits of the venous sinus of spleen red pulp, both of which are narrower than their smallest dimension.1 This necessitates maintenance of the ability of RBCs to undergo repeated, extensive, and reversible deformations. Repeated major membrane deformations induce ion and water permeability changes in the RBCs.2-5 The biconcave discoid shape endows the human RBC with an advantageous surface area-to-volume (S/V) ratio, allowing the cell to undergo marked deformations while maintaining a constant surface area.6-8
A reduced RBC S/V ratio has long been recognized to contribute to pathogenesis of several RBC disorders,9-11 including hereditary spherocytosis (HS), the most common cause of inherited chronic hemolytic anemia in Northern Europe and North America, with an estimated incidence of 1 in 2000.11 The clinical presentation of HS can range from mild to severe hemolytic anemia.12,13 The molecular basis of HS is heterogeneous, the common denominator being the loss of HS RBC membrane surface area due to specific molecular defects in several membrane proteins (α or β spectrin, ankyrin, protein 4.2, and protein band 3), which result in the loss of cohesion between the lipid bilayer and the membrane skeleton.10,11,14,15 The loss of membrane surface area results in the transformation of the biconcave discoid shape, first to a stomatocyte and finally to a spherocyte, with a progressive reduction in cellular deformability.
Although a major role for the spleen in pathogenesis of HS is well established,16 there are currently no data on the quantitative relationship between the extent of surface area loss and the extent of splenic entrapment. The only indirect evidence comes from early studies documenting reduced survival of Cr51-labeled spherocytes infused into healthy recipients, whereas the survival of normal RBCs in HS subjects was normal.17-19 This implied that the reduced life span and splenic entrapment was an intrinsic feature of HS RBCs. Unfortunately, because neither the surface area nor the S/V ratio of infused spherocytes was measured in these studies, the extent of membrane surface area loss (or reduced S/V ratio) that leads to splenic retention of altered RBCs remains undefined. As a result, there is no predictive biologic parameter with which to estimate the risk of splenic entrapment of spherocytic cells and the ensuing anemia. Previously, we addressed this important issue using the isolated human spleen system20 perfused with human RBCs with defined extents of surface area loss.
RBCs with defined loss in membrane surface area can be generated experimentally by treatment with lysophosphatidylcholine (LPC).6,7 By initially accumulating exclusively in the external leaflet of the RBC lipid bilayer, LPC induces in a dose-dependent manner echinocytosis (spiculation) and eventually spherocytes through the release of microvesicles.21,22 The effects of LPC on RBC morphology, surface area loss and S/V ratio,7,8,21,22 and cellular deformability6,7,23,24 have been documented extensively. In the present study, we used LPC treatment to induce various degrees of surface area loss and assessed several cellular changes: cell shape, S/V ratio, and cell deformability. The ability of the treated RBCs to traverse human splenic sinuses was monitored using the isolated perfused spleen system20 and the rate of sequestration was quantified. We have established herein for the first time that RBCs with an average surface area loss of > 18% (corresponding to a > 27% decrease of S/V ratio) are rapidly sequestered in the spleen. Unexpectedly, kinetic analysis showed that a subset of RBCs with < 18% surface area loss adapted to repeated passages through the spleen by decreasing their volume and thus their sphericity, thereby escaping splenic entrapment.
Methods
This study was approved by the investigational review board of Ile-de-France II (Paris, France).
Human spleen retrieval
Spleens were retrieved and processed as described previously.20,25,26 Medical and surgical care were not modified, and patient written consent was obtained. All patients (5 female and 4 male, 47-72 years of age) underwent left splenopancreatectomy for pancreatic disease (neuroendocrine tumor, proven or suspected adenocarcinoma, cyst, unspecified tumor, or chronic pancreatitis). Spleens were macroscopically and microscopically normal in all cases. After examination of the macroscopic aspect of the spleen by the pathologist during a 30-minute period of warm ischemia linked to the surgical procedure, the main splenic artery was cannulated. The spleens were flushed with cold RPMI 1640-albumin solution for transport to the laboratory.
RBC labeling with PKH67 and/or PKH26
Blood from a blood center (Etablissement Français du Sang, Rungis, France) was washed 3 times in RPMI 1640 to remove WBCs. RBCs were labeled with the lipophilic fluorescent probes PKH67 or PKH26 (hematocrit, 5%) according to the directions of the manufacturer (Sigma-Aldrich) with some modifications to obtain 4 different populations of labeled RBCs. Two populations of RBCs were labeled with one PKH (RBC/PKH67, PKH67 dilution 1/1000; RBC/PKH26, PKH26 dilution 1/250). Two other populations of RBCs were labeled with both PKHs at different concentrations (RBC/KH26-67, PKH67 dilution 1/2000 plus PKH26 dilution 1/500; RBC/PKH67-26, PKH67 dilution 1/1000 plus PKH26 dilution 1/3000). These 4 populations of labeled RBCs could be distinguished by FACS, which allowed us to perfuse 4 distinct preparations in a single spleen and trace each population individually.
Treatment of RBCs with LPC
PKH-labeled RBCs were resuspended in LPC (0-15μM) in PBS or PBS alone at a 1% hematocrit level to induce a controlled loss of membrane area. The LPC samples were incubated for 5 minutes at room temperature. After incubation, samples were washed 3 times with PBS and resuspended in Krebs-albumin for further analysis.
Measurement of RBC deformability
RBC deformability was measured by ektacytometry using a laser-assisted optical rotational cell analyzer (Mechatronics) as described previously.27 The elongation index (EI), a unit of RBC deformability, was defined as the ratio of the difference between the 2 axes of the ellipsoidal diffraction pattern and the sum of these 2 axes.
Ex vivo spleen perfusion
Isolated-perfused spleen experiments were performed as described previously20,25 in a Plexiglas chamber maintained at 37°C by a regulated warm air flow. The perfusion of mixture of untreated (RBC/PKH) or LPC-treated (RBC/PKH/LPC) PKH-labeled RBCs (15%-25% final hematocrit in Krebs-albumin medium) was performed over a 1- to 2-hour period. The percentage of circulating LPC-treated cells was quantified by flow cytometric analysis (FACSCalibur; BD Biosciences). Data were analyzed using CellQuest 3.3 software (BD Biosciences).
Analysis of RBC morphology and dimensions
Untreated or LPC-treated RBCs were fixed with PBS-paraformaldehyde (1%) for analysis. Image acquisition and data analysis were done as described previously.28 Images were acquired on the ImageStream imaging cytometer (Amnis). At least 10 000 images were collected for each sample. Postacquisition data analysis was performed using the IDEAS Version 4.0 image analysis software package (Amnis). Morphologic (compactness, circularity, aspect ratio, and shape ratio) and dimension (surface area, diameter, and perimeter) parameters of RBCs were calculated using images of RBCs by IDEAS software.
Osmotic fragility test
Osmotic fragility of RBCs was determined according to the method originally described by Parpart et al.29 Untreated or LPC-treated RBCs were incubated for 30 minutes in hypotonic solutions with NaCl content ranging from 0.1%-0.9% (hematocrit, 1%). After centrifugation, absorbance of the supernatants was measured at 540 nm using a spectrophotometer and the percent hemolysis was calculated for each supernatant and plotted against NaCl concentrations.
Scanning electron microscopy
RBC specimens were fixed in 2.5% glutaraldehyde in cacodylate buffer (0.1M) overnight at 4°C. RBCs were dropped gently onto a glass coverslip that had been treated with polylysine. After 3 cacodylate buffer rinses (10 minutes each), the specimens were fixed with 1% osmium tetroxide for 1 hour. After 3 rapid washes in water, samples were processed through an ethanol dehydration series of 25%-100% ethanol, followed by critical point drying with CO2. Dried specimens were sputtered with 22-nm gold palladium, examined, and photographed with a JEOL JSM 6700F field emission scanning electron microscope operating at 5 or 7 kV. Images were acquired with the upper detector and the lower secondary detector.
Results
RBC treatment with LPC results in a dose-dependent loss of surface area and deformability
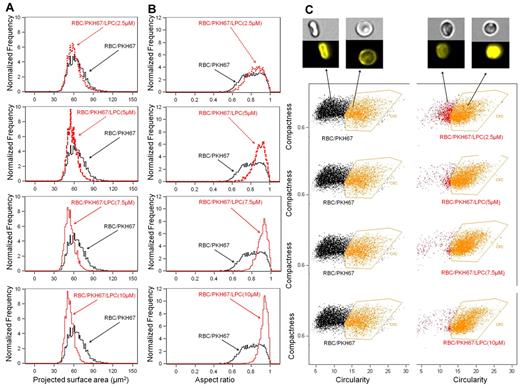
RBCs were exposed to increasing LPC concentrations to generate RBC populations with progressively decreasing membrane surface areas (Figure 1A). Surface areas and diameters of the LPC-treated RBCs were reduced significantly (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and their volume was only slightly elevated (Table 1), resulting in a substantial increase in sphericity, with most cells becoming nearly perfect spheres at high LPC concentrations (Figure 1B and supplemental Figure 1B-D). The RBC populations were morphologically homogenous even at low LPC concentrations, as indicated by the uniform shifts of RBC distribution patterns (Figure 1C). Because of the decrease in RBC surface area and a corresponding slight volume increase, there was a decrease of the S/V ratio (Table 1). RBCs with a decreased S/V ratio exhibit increased osmotic fragility in hypertonic solution,7 a feature displayed by the LPC-treated RBCs (Figure 2A-B).
Effects of LPC treatment on RBC membrane surface area and cell morphology. Analysis of RBC dimension, morphology, and morphologic homogeneity using ImageStream technology. There is a dose-dependent shift toward lower values of the distribution of RBC membrane surface area (A), whereas the aspect ratio (sphericity; B) increased with increasing concentrations of LPC. (C) The RBC populations are morphologically homogenous even at low LPC concentrations, as characterized by the RBC distribution shifting as a whole. RBCs with a circular shape in the control group did not correspond to spherical cells, but were instead discocytes, because the images were captured face on.
Effects of LPC treatment on RBC membrane surface area and cell morphology. Analysis of RBC dimension, morphology, and morphologic homogeneity using ImageStream technology. There is a dose-dependent shift toward lower values of the distribution of RBC membrane surface area (A), whereas the aspect ratio (sphericity; B) increased with increasing concentrations of LPC. (C) The RBC populations are morphologically homogenous even at low LPC concentrations, as characterized by the RBC distribution shifting as a whole. RBCs with a circular shape in the control group did not correspond to spherical cells, but were instead discocytes, because the images were captured face on.
Characteristics of untreated RBCs and RBCs treated with increasing concentrations of LPC (1-15μM)
| Sample . | Hb, g/dL . | MCHC, g/dL . | MCH, pg . | MCV, μm3 . | Projected area, μm2 . | A/V, μm−1 . | Sphericity . | EImax, a.u. . | Spleen retention, % . |
|---|---|---|---|---|---|---|---|---|---|
| Untreated RBCs | 11.3 (1.8) | 34 (2) | 31 (3) | 92 (2) | 71 (1) | 0.77 (0.01) | 0.81 (0.01) | 0.638 (0.01) | 9 (4) |
| LPC-treated RBCs | |||||||||
| 1-2.5μM | 10.5 (0.8) | 35 (2) | 32 (3) | 92 (1) | 70 (0.3) | 0.76 (0.01) | 0.81 (0.02) | 0.621 (0.01) | 19 (7) |
| 3.5-5.0μM | 10.1 (0.3) | 35 (1) | 33 (1) | 94 (0) | 63 (3) | 0.67 (0.03) | 0.91 (0.03) | 0.520 (0.04) | 64 (16) |
| 6.0-7.5μM | 9.7 (0.2) | 33 (2) | 32 (1) | 97 (1) | 59 (3) | 0.61 (0.04) | 0.92 (0.02) | 0.470 (0.05) | 79 (13) |
| 8.5-15.0μM | 10.1 (0.1) | 32 (1) | 30 (2) | 95 (0) | 56 (3) | 0.59 (0.03) | 0.93 (0.01) | 0.423 (0.04) | 92 (4) |
| Sample . | Hb, g/dL . | MCHC, g/dL . | MCH, pg . | MCV, μm3 . | Projected area, μm2 . | A/V, μm−1 . | Sphericity . | EImax, a.u. . | Spleen retention, % . |
|---|---|---|---|---|---|---|---|---|---|
| Untreated RBCs | 11.3 (1.8) | 34 (2) | 31 (3) | 92 (2) | 71 (1) | 0.77 (0.01) | 0.81 (0.01) | 0.638 (0.01) | 9 (4) |
| LPC-treated RBCs | |||||||||
| 1-2.5μM | 10.5 (0.8) | 35 (2) | 32 (3) | 92 (1) | 70 (0.3) | 0.76 (0.01) | 0.81 (0.02) | 0.621 (0.01) | 19 (7) |
| 3.5-5.0μM | 10.1 (0.3) | 35 (1) | 33 (1) | 94 (0) | 63 (3) | 0.67 (0.03) | 0.91 (0.03) | 0.520 (0.04) | 64 (16) |
| 6.0-7.5μM | 9.7 (0.2) | 33 (2) | 32 (1) | 97 (1) | 59 (3) | 0.61 (0.04) | 0.92 (0.02) | 0.470 (0.05) | 79 (13) |
| 8.5-15.0μM | 10.1 (0.1) | 32 (1) | 30 (2) | 95 (0) | 56 (3) | 0.59 (0.03) | 0.93 (0.01) | 0.423 (0.04) | 92 (4) |
All data are means (SD). Hb, MCHC, MCH, and MCV were measured with ADVIA 120 Hematology System.
Hb indicates hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCH, mean corpuscular hemoglobin; MCV, mean cell volume; EImax, EI at a shear stress of 30 Pascals; and a.u., arbitary units.
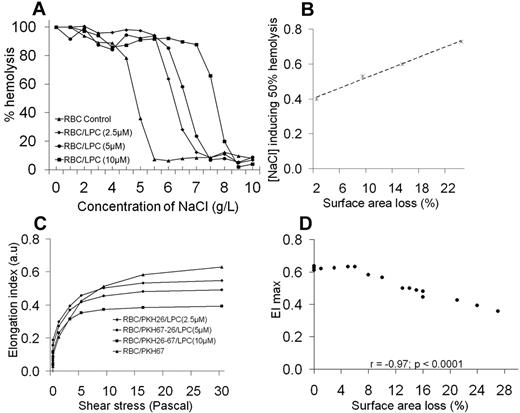
Monitoring of LPC-treated RBC features. (A) Osmotic fragility test of LPC-treated RBCs (1 of 3 representative experiments). (B) Linear regression fit of the correlation between NaCl concentration inducing 50% of hemolysis and the extent of surface area loss (a representative experiment). There was a good correlation (r = 0.998; P < .0001) between the extent of surface area loss and the osmolarity value at which 50% of RBCs hemolyze. (C) Deformability profiles of LPC-treated RBCs used for splenic perfusion studies (a representative experiment). The RBC deformability changes that occurred after LPC treatment were examined with the laser-assisted optical rotational cell analyzer, a system that measures the extent of cell deformation as a function of applied shear stress. LPC-treated RBCs were less deformable than control/untreated RBCs, with a dose dependent reduction in EImax, a deformability parameter. (D) Linear regression fit of the correlation between EImax and the extent of surface area loss.
Monitoring of LPC-treated RBC features. (A) Osmotic fragility test of LPC-treated RBCs (1 of 3 representative experiments). (B) Linear regression fit of the correlation between NaCl concentration inducing 50% of hemolysis and the extent of surface area loss (a representative experiment). There was a good correlation (r = 0.998; P < .0001) between the extent of surface area loss and the osmolarity value at which 50% of RBCs hemolyze. (C) Deformability profiles of LPC-treated RBCs used for splenic perfusion studies (a representative experiment). The RBC deformability changes that occurred after LPC treatment were examined with the laser-assisted optical rotational cell analyzer, a system that measures the extent of cell deformation as a function of applied shear stress. LPC-treated RBCs were less deformable than control/untreated RBCs, with a dose dependent reduction in EImax, a deformability parameter. (D) Linear regression fit of the correlation between EImax and the extent of surface area loss.
Ektacytometric analysis showed that LPC-treated RBCs exhibited lower cellular deformability than control RBCs (Figure 2C), a feature that was not related to increased internal RBC viscosity,7 because there was little or no change in the mean corpuscular hemoglobin concentration and thus cytoplasmic viscosity after exposure to LPC (Table 1). The deformability profiles were characteristic of RBCs exhibiting a loss of surface area,7 implying that a reduced RBC S/V ratio accounts for decreasing values of EI (a deformability parameter) of LPC-treated RBCs. The maximum value of EI (EImax) and the percentage of surface area loss were correlated inversely (r = −0.97; P < .0001; Figure 2D). LPC-treated RBCs do not mimic HS RBCs perfectly. In addition to surface area loss, there is the additional contribution of increased internal viscosity to reduced deformability of HS RBCs because of the presence of a subpopulation of dehydrated cells with high mean corpuscular hemoglobin concentration.6
Retention of LPC-treated RBCs by the isolated-perfused human spleen is determined by the extent of surface area loss
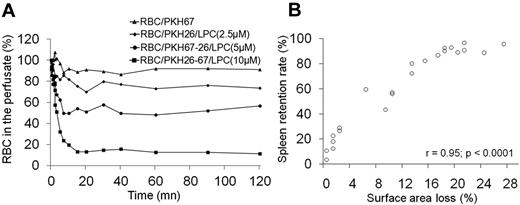
To understand how the human spleen handles RBCs displaying decreased surface area at constant mean cell volume (ie, with a reduced S/V ratio), isolated human spleens were perfused with a preparation containing RBCs exposed to different LPC concentrations and control RBCs, each differentially labeled with fluorescent PKH26 and/or PKH67 probes. Circulating cells were sampled from the perfusion medium over a 2-hour period of perfusion and the various labeled RBC populations were monitored by flow cytometry. The LPC-treated RBCs were rapidly cleared by the human spleen, with a mean clearance half-time of 3.5 minutes (range, 2.0-5.0; Figure 3A and supplemental Figure 2). The maximum level of clearance occurred between 10 and 20 minutes. The level of splenic retention increased (range, 18%-96%) with increasing concentrations of LPC (0-10μM; Figure 3A) and with increasing extents of surface area loss (range, 1%-28%; Figure 3B). There was a positive correlation (r = 0.95; P < .0001) between the percentage of RBCs retained by the spleen and the extent of RBC surface area loss. A surface area loss of 3% (an approximately 13% decrease of the S/V ratio) resulted in the retention of 27% of RBCs within the spleen (Table 1). Clearance of the RBC population became massive (> 90%) after a surface area loss of > 18% (> 27% decrease of the S/V ratio; Figure 3B). This retention cannot be explained by saturation of the splenic filtration function, given the small percentage of modified RBCs perfused (approximately 4%-5% of the total RBCs).
Clearance of LPC-treated RBCs by isolated perfused human spleens. (A) Data from a representative experiment showing kinetics of circulating LPC-treated RBCs recovered from the perfusate over 2 hours of splenic perfusion. RBCs were differentially labeled with the lipophilic fluorescent probes PHK26 and/or PKH67, enabling the distinction of 4 separate LPC-treated RBC populations by flow cytometry. (B) Linear regression fit of the correlation between EImax and the level of LPC-treated RBC retention within the spleen (n = 9 independent experiments).
Clearance of LPC-treated RBCs by isolated perfused human spleens. (A) Data from a representative experiment showing kinetics of circulating LPC-treated RBCs recovered from the perfusate over 2 hours of splenic perfusion. RBCs were differentially labeled with the lipophilic fluorescent probes PHK26 and/or PKH67, enabling the distinction of 4 separate LPC-treated RBC populations by flow cytometry. (B) Linear regression fit of the correlation between EImax and the level of LPC-treated RBC retention within the spleen (n = 9 independent experiments).
Adaptation of a subset of surface-deficient RBCs during splenic transit
Even at the highest extent of membrane surface area loss, a subset of LPC-treated RBCs exited from the spleen, as reflected by the existence of a plateau in cell clearance studies (Figure 3A). To obtain insights into the cellular features of this subpopulation, we compared the dimension and morphology of the RBCs at the onset of perfusion (time 0, T0) and those recovered from the perfusate after 40 minutes of perfusion (T40; ie, when the plateau was attained). Data from a representative study are shown in Figure 4 and supplemental Figure 3A through D. The dimensions and morphologic parameters of untreated RBCs were unchanged between T0 and T40 (supplemental Figure 3C-D). For all concentrations of LPC, surface area, diameter, and perimeter of the LPC-treated RBCs were similar at T0 and T40 and were significantly lower than that of untreated RBCs (Figure 4A-C). In marked contrast, the circularity and aspect ratio values of the LPC-treated RBCs collected at T40 were different from values at T0 and closer to those of control RBCs (Figure 4D-E). These findings suggest a likely reduction in the volume of a subpopulation of LPC-treated RBCs circulating at T40 to compensate for the surface area loss. The acquisition of a more favorable S/V ratio due to decreased volume enabled the sustained circulation of this population of cells with reduced surface area.
Adaptation and survival of RBCs with reduced surface area and S/V ratio during the isolated-human spleen perfusion. Dimension (A-C) and morphology (D-E) analysis of LPC-treated RBCs before (T0) and 40 minutes (T40) after the start of the perfusion. Projected surface area (A), diameters (B), and perimeters (C) of LPC-treated RBCs were similar between T0 and T40, and all were significantly lower than that of untreated RBCs. Shifts toward normal values of the circularity (D) and the aspect ratio or sphericity (E) of LPC-treated RBCs at T40. See also supplemental Figure 3 for the deformability profile of LPC-treated RBC populations before and the kinetics of their clearance during 60 minutes of spleen perfusion. Projected surface area is in square micrometers; diameter and perimeter are in micrometers. One of 3 representative experiments is shown.
Adaptation and survival of RBCs with reduced surface area and S/V ratio during the isolated-human spleen perfusion. Dimension (A-C) and morphology (D-E) analysis of LPC-treated RBCs before (T0) and 40 minutes (T40) after the start of the perfusion. Projected surface area (A), diameters (B), and perimeters (C) of LPC-treated RBCs were similar between T0 and T40, and all were significantly lower than that of untreated RBCs. Shifts toward normal values of the circularity (D) and the aspect ratio or sphericity (E) of LPC-treated RBCs at T40. See also supplemental Figure 3 for the deformability profile of LPC-treated RBC populations before and the kinetics of their clearance during 60 minutes of spleen perfusion. Projected surface area is in square micrometers; diameter and perimeter are in micrometers. One of 3 representative experiments is shown.
Discussion
The results of the present study demonstrate clearly that cell surface area loss with reduced S/V ratio is a significant predictor of splenic sequestration of human RBCs. A loss of 18% of the cell surface area (corresponding to a > 27% decrease of the S/V ratio) led to rapid RBC entrapment in normal human spleens. The majority of these surface-deprived RBCs were trapped during their first passage through the isolated spleen. Therefore, it appears that their spheroidal shape renders them incapable of undergoing the cellular deformation necessary to squeeze across the inter-endothelial slits of the venous sinus, which have apertures of 0.2-0.5 × 2-3 μm.1,26 This determination of human spleen retention threshold of surface-deficient RBCs has significant implications for diagnosis (spleen functionality) or prognosis studies in the field of RBC hemolytic disorders and for drug screening (identification of compounds susceptible to inducing severe anemia).
In the present study, we found that even at the highest extent of membrane surface area loss, a subset of LPC-treated RBCs exited from the spleen. Several hypotheses might explain these results. First, the heterogeneity of the initial population of LPC-treated RBCs might include more spherical RBCs (preferentially entrapped in the spleen) and LPC-treated RBCs with dimensions closer to normal values. The fact that LPC-treated RBC dimensions (for all concentrations of LPC) were similar between T0 and T40 and were both reduced significantly from that of untreated RBCs (Figure 4A-C) exclude the possibility that heterogeneity of the initial LPC-treated RBC population is responsible for continued circulation of this subpopulation of cells. Second, LPC-treated RBCs might acquire membrane lipids30 that increase their surface area and restore the cell S/V ratio; permitting them to escape spleen retention.31 In the present study, all experiments were done without serum or plasma. Moreover, the fact that LPC-treated RBCs dimensions were similar between T0 and T40 and were both different from that of control RBCs (Figure 4A-C) suggests that the reacquisition of membrane surface area by LPC-treated RBC membrane cannot explain the presence of an uncleared LPC-treated RBC subpopulation. Third, the survival of an LPC-treated RBC subpopulation might be because of their S/V ratio returning toward normal values through a decrease in cell volume as a result of regulation of ions and water permeability of the cell.2-5
It is well established that, when submitted to increased mechanical stress, normal RBCs exhibit a reversible increase in permeability to monovalent2-5,32,33 and divalent5,34 cations and to anions.5 Such phenomena have been shown to be exacerbated when RBC membrane rigidity is increased3 or when the surface area is reduced, as is the case in HS RBCs.14,35 This provokes a transient dehydration and increased loss of intracellular potassium that is only partially offset by an increase in intracellular sodium.14,34,36,37 If such a process takes place in the circulating LPC-treated RBCs, this would lead to a decrease in cell volume, creating a more favorable S/V ratio and enabling the surface-deficient RBCs to remain in the circulation. This hypothesis is supported by the fact that LPC-treated RBC dimensions were similar at T0 and T40 and both were significantly lower than that of untreated RBCs (Figure 4A-C). However, the sphericity value of untrapped, LPC-treated RBCs was different from values at T0 (Figure 4D-E) and closer to those of control RBCs (supplemental Figure 3C-D). This phenomenon of adaptive volume regulation might take place between 10 and 20 minutes of splenic perfusion, indicating that acquisition of a favorable S/V ratio by the surface-deficient RBC subpopulation takes a relatively short time, possibly through deformation-induced membrane permeability modifications. Similar findings have been described previously in a study in mice,8 which have nonsinusoidal spleens.38
In summary, the present study enabled the definition of the relationship between the S/V ratio of human RBCs and splenic entrapment and documented the critical extent of surface area loss that leads to rapid splenic removal. Interestingly, it provides a novel paradigm, namely that surface-deprived RBCs might adapt and escape splenic retention through the regulation of cell volume. The human RBC plasma membrane and the membrane skeleton provide the RBCs with unique structural and functional properties, permeability characteristics that operate to regulate a favorable S/V ratio necessary for undergoing extensive deformations essential for optimal function.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Pamela Tamez for the critical review of the manuscript. N.M. acknowledges the “Fonds dédié Combattre les maladies parasitaires,” Sanofi Aventis–Ministère de l'Enseignement Supérieur et de la Recherche–Institut Pasteur, which made possible his sabbatical stay at Institut Pasteur, and funding from the National Institutes of Health (DK26263 and HL31579).
This work was supported by the “Fonds dédié Combattre les maladies parasitaires,” Sanofi Aventis–Ministère de l'Enseignement Supérieur et de la Recherche–Institut Pasteur, the Center National de la Recherche Scientifique, and the Institut Pasteur. I.S. was funded by a fellowship from the Région Ile de France. G.D. was supported by the Délégation Générale à l'Armement (fellowship 0560-00-032) and the Région Ile de France. The research leading to these results was funded by the European Union Seventh Framework Program (FP7/2007-2013) under grant agreement 242095 (Evimalar), by the project “Mechanisms of Erythrocytic Infection and Anemia in Malaria” (principal investigator, Kasturi Haldar), and by the National Institutes of Health (5P01HL078826-06). The ImageStream apparatus (Amnis) was acquired by a grant from the Conseil de la Region Ile de France program Sesame 2007 (number I-08-1090 Imagopole).
National Institutes of Health
Authorship
Contribution: I.S. and P.A.B. designed and performed the research, contributed vital analytical tools, analyzed the data, and wrote the manuscript; G.D. and S.P. designed and performed the research, contributed vital analytical tools, and analyzed the data; V.B. designed and performed the research and analyzed the data; A.N. and M.N. performed the research and analyzed the data; and O.M.-P., P.H.D., G.M., and N.M. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for I.S. is Department of Biological Sciences, University of Notre Dame, Notre Dame, IN.
Correspondence: Innocent Safeukui, Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556; e-mail: innocent.safeukui@nd.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal