Abstract
Follicular lymphoma is a monoclonal B-cell malignancy with each patient's tumor expressing a unique cell surface immunoglobulin (Ig), or B-cell receptor (BCR), that can potentially recognize antigens and/or transduce signals into the tumor cell. Here we evaluated the reactivity of tumor derived Igs for human tissue antigens. Self-reactivity was observed in 26% of tumor Igs (25 of 98). For one follicular lymphoma patient, the recognized self-antigen was identified as myoferlin. This patient's tumor cells bound recombinant myoferlin in proportion to their level of BCR expression, and the binding to myoferlin was preserved despite ongoing somatic hypermutation of Ig variable regions. Furthermore, BCR-mediated signaling was induced after culture of tumor cells with myoferlin. These results suggest that antigen stimulation may provide survival signals to tumor cells and that there is a selective pressure to preserve antigen recognition as the tumor evolves.
Introduction
Follicular lymphoma (FL) is a slowly progressive and largely incurable human B-cell malignancy. Transformation to a more aggressive lymphoma, such as diffuse large B-cell lymphoma, is common and strongly associated with an increase in morbidity and mortality. A chromosomal translocation t(14:18) is the hallmark of this disease, and it is found in 85%-90% of cases. It results in the juxtaposition of the BCL2 proto-oncogene with the immunoglobulin (Ig) heavy chain gene, IGH, leading to deregulated overexpression of Bcl-2 protein, a major inhibitor of apoptosis. However, the t(14:18) translocation is insufficient to cause malignancy as it is detectable in rare B cells from healthy persons.1-3 Thus, FL pathogenesis requires additional signals beyond that imparted by the deregulation of BCL2. The observation that FL cells isolated from patients fail to survive in vitro and undergo spontaneous apoptosis supports the hypothesis that extrinsic microenvironmental factors are required for maintenance and expansion of FL.4
Phenotypically, FL tumor cells resemble antigen-experienced germinal center B cells. Their Ig genes, which are rearranged to produce a functional B-cell receptor (BCR), have numerous point mutations compared with their germline counterparts, and this process of somatic hypermutation (SHM) is ongoing as the malignant clone expands and diversifies. Thus, individual tumor cells can each have slightly different Ig variable region sequences.5 Random mutations should eventually result in stop codons and loss of BCR protein expression. However, FL tumors maintain a surface BCR, indicating a selective force favoring retention of a functional BCR. Furthermore, therapy with anti-idiotype antibodies directed against the BCR did not select for the outgrowth of BCR-negative variants. Rather, this therapy selected for the outgrowth of cells that had amino acid substitutions in the targeted V region sequence, making them unrecognizable by the anti-idiotype antibody.6 Other in vitro studies with malignant B-cell lines have shown that experimental knockdowns of the BCR and members of its signaling pathway result in growth arrest, implicating their importance in tumor cell survival.7
The BCR can transmit a tonic survival signal, but this is greatly augmented on its binding to a cognate antigen.8 There is indirect evidence to suggest that antigen recognition plays a role in the pathogenesis of FL. SHM can introduce silent or replacement mutations, the latter leading to an amino acid substitution. In a normal immune response, B cells with mutations resulting in higher binding affinity for the inciting antigen preferentially survive. This selective pressure leads to enrichment of replacement mutations in the complementarity determining regions (CDRs) of the BCR, and an under representation of replacement mutations in the framework regions (FWRs).9 This same distribution of replacement and silent mutations has been reported for the BCRs of FL cells,5 and the intraclonal diversity resulting from ongoing SHM argues for the existence of an antigen driving the growth of the tumor.10,11 However, improved methods for assessing antigen-driven selection revealed strong negative selection against replacement mutations in the FWRs, but no positive selection in the CDRs of FL Ig variable regions.12,13 These findings gave evidence for a selective pressure that maintains the expression of a functional BCR, but not for antigen recognition.
Nevertheless, there are severalexamples of antigen recognition by B-cell malignancies. A case of lymphoma arising in a patient infected with hepatitis C virus had a tumor BCR that bound the viral envelope protein.14 Furthermore, in some patients with both hepatitis C virus infection and lymphoma, antiviral therapy led to regression of the lymphoma,15 indicating a dependence of the lymphoma on the continued presence of the virus. Mucosa-associated lymphoid tissue lymphomas have been reported to bind bacterial antigens as well as self-antigens, including Ig, DNA, and stomach extracts.16,17 In addition, a subset of splenic marginal zone lymphomas using the IgH VH1-02 gene, have similar CDRs and were shown to be both poly- and self-reactive.18 In chronic lymphocytic leukemia (CLL), 30% of patients use a restricted Ig repertoire, have stereotypic BCR sequences, and are frequently self-reactive.19-21 These analyses suggest that shared antigens may select tumor cell progenitors. Indeed, a subset of unmutated CLL tumors with stereotyped BCRs were shown to bind a self-antigen identified as nonmuscle myosin heavy chain II.21 Unlike CLL, FL tumors do not have biased Ig V gene usage or stereotypic Ig V regions sequences.22 However, some FL BCRs have been reported to be reactive with self-antigens.23 These results provide some support for the hypothesis that self-antigens provide survival signals to FL tumor cells.
To investigate the hypothesis that self-antigen recognition plays a role in the pathogenesis of FL, we used recombinant tumor Igs, which had been produced for a clinical trial of therapeutic vaccination (NCT00017290),24 and evaluated the reactivity of tumor derived Igs for human tissue antigens. Furthermore, we used a self-antigen recognized by the BCR from a patient's tumor to perform a clonal analysis of antigen recognition and tested the ability of cognate antigen to transduce signals through the tumor BCR.
Methods
Recombinant tumor Ig
Recombinant tumor Igs were cloned as part of a clinical trial from patients with previously untreated advanced stage FL25,26 and generated as previously described.24 The tumor Ig DNA and protein for each patient are identified with a 4-digit numerical identifier. Variable regions were sequenced and analyzed for Ig gene usage and for the number of replacement and silent mutations with IMGT V-Quest. The focused binomial test was used to ascertain selection (ie, the probability that an excess or scarcity of replacement mutations in the V gene CDRs or FWRs) occurred by chance.27
Cell lines secreting recombinant tumor Ig were grown in protein-free RPMI with 1mM sodium pyruvate, nonessential amino acids, 100 μg/mL streptomycin, 100 U/mL penicillin G, 2mM l-glutamine (Invitrogen), and 1% Nutridoma-SP (Roche Applied Science). To generate Igs lacking N-linked glycans, cells were grown in media supplemented with 2 μg/mL tunicamycin (Roche Diagnostics). Supernatants were harvested, and Ig was purified using protein G followed by elution with 0.1M glycine (pH 3.0) and neutralization with 1M Tris (pH 8.0). Concentrations of recombinant tumor Ig were determined by ELISA and by A280 absorbance using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific).
Analysis of N-linked glycosylation
Recombinant tumor Igs were treated separately with Endo-H and PNGase F (New England BioLabs) according to the manufacturer's instructions. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed with anti–human IgG-HRP (Southern Biotech 2040-05). Increased mobility after treatment with Endo H or PNGase F indicates the presence of oligomannose glycans or N-linked glycans, respectively.
IFA
Tumor Igs were tested for self-reactivity by indirect immunoflurescence assay (IFA) on HEp-2 cells (Bion Enterprises) according to the manufacturer's instructions. Igs were diluted in PBS with 2% BSA and used at 90-150 μg/mL. Anti–human Ig-FITC detected binding of tumor Igs. Evans Blue was used as a counterstain. Staining was performed under moist conditions at room temperature. Slides were imaged with a Nikon Eclipse E800 microscope with a Nikon Plan Apochromatic 1.40 aperture 100× objective lens, for a final magnification of 1000×.
Expression of recombinant myoferlin
Recombinant myoferlin containing a C-terminal HA tag was kindly provided by Dr William Sessa28 (Addgene). Recombinant myoferlin fused to the mouse IgG2a Fc was generated by cloning myoferlin cDNA into pFUSE-mIgG2A-Fc2 expression vector (InvivoGen). Recombinant myoferlin cDNA was transfected into 293T cells with Fugene HD (Promega) as per the manufacturer's instructions. At 24 hours after transfection, cells were harvested. Clarified lysates were used in immunoprecipitations and ELISAs. In flow cytometry-based staining and stimulation assays, detergent was adsorbed with BioBeads SM-2 Adsorbent (Bio-Rad).
Immunoprecipitation, immunoblot, and protein identification
Cells were lysed at 10 × 106 cells/mL with nondenaturing lysis buffer (20mM Tris HCL pH 8, 137mM NaCl, 1% NP-40) containing protease inhibitors (Roche cOmplete Mini, EDTA-free protease inhibitor tablets) at 4°C for 45 minutes followed by centrifugation at 20 000g for 20 minutes at 4°C; 1 μg of tumor Ig was added to 1 mL of lysate and rotated for 2 hours at room temperature, followed by addition of 25 μL of protein G beads (Dynabeads, Invitrogen), and continued rotation for 15 minutes. The beads were washed 5× with PBS and samples were eluted with nonreducing SDS sample buffer, and separated by SDS-PAGE. Gels were silver-stained for mass spectrometry (Pierce, Thermo Scientific) or transferred to nitrocellulose membrane for immunoblots.
Membranes were probed with mouse antimyoferlin (Novus Biologicals, H00026509) followed by detection with HRP-conjugated goat anti–mouse IgG (Southern Biotechnology, 1030-05). Blots were developed with ECL Western blotting detection reagent (GE Healthcare). For mass spectrometry analysis, gel pieces containing silver-stained proteins were subjected to in-gel tryptic digestion (Pierce, Thermo Scientific) and identified by LC-MS/MS using the Agilent 1100 LC system and the Agilent XCT plus Ion Trap (Agilent Technologies), as previously described.29 The MS/MS spectra were scanned against the SwissProt database using the SpectrumMill software (Agilent).
Myoferlin ELISA
The 96-well flat-bottom plates were coated with 5 μg/mL goat anti-HA (Abcam ab9134), followed by blocking with 5% milk in PBS and incubation with lysate of 293T cells transfected to express recombinant myoferlin protein. Lysates of untransfected cells served as a negative control. Plates were probed with 10 μg/mL of tumor Ig diluted 3-fold in 2% BSA in PBS. Binding of tumor Ig to myoferlin was detected with goat anti–human IgG-HRP (Southern Biotechnology). ELISAs were developed with ABTS (Sigma-Aldrich) and read with a Vmax kinetic microplate reader (Molecular Devices).
Biolayer interferometry
Equilibrium affinity measurements were performed using an Octet QK (Foretebio) at 25πC at 1000rpm.30 Biotinylated goat anti-HA (GenScript, A00203) was loaded onto streptavidin-coated sensor tips (Fortebio) followed by capture of recombinant myoferlin-HA protein from lysate of 293T cells transfected with recombinant myoferlin cDNA. Real-time interactions between surfaces with immobilized myoferlin and different concentrations of tumor Ig 1152 were measured simultaneously for 18 000 seconds using a separate sensor tip for each concentration condition. Interactions were monitored until at or near equilibrium. The equilibrium affinity (KD) of myoferlin/tumor Ig 1152 was approximated by fitting a plot of final signal intensity versus tumor Ig 1152 concentration to a 1:1 binding model, using GraphPad Prism Version 5.0.
Intraclonal tumor Ig VH diversity
DNA was extracted from the biopsy of patient 1152 (AllPrep DNA/RNA Micro Kit, QIAGEN) was amplified using PHusion High-fidelity PCR Kit (New England BioLabs). Primers matched the 5′ region of FWRs1 (5′-CAGGTCACCTTGAGGGAGTCTGG-3′) and the 3′ region of FWRs4 (5′-TGAGGAGACGGTGACCAGGG-3′). PCR product was ligated into Zero Blunt PCR Cloning vector (Invitrogen) and transformed into competent Escherichia coli OneShot TOP10 cells. Plasmids isolated from single colonies were sequenced using M13 universal primers. Sequences were aligned with MacVector 12 software. To estimate false mutations introduced by experimental methods, we cloned and sequenced the VH from this patient's B cell hybridoma (6C12). A single mutation was present in the 29 molecular clones sequenced, indicating a false mutation rate of 0.03 mutations/VH.
To rescue the soluble Ig from single tumor cells of patient 1152, the cells were fused to K6H6/B5 heteromyeloma at a ratio of 1:1 with polyethylene glycol and cultured in the presence of 100μM hypoxanthine, 0.4μM aminopterin, 16μM thymidine (Sigma-Aldrich). Hybridoma clones that were positive in a screen for Ig-λ were further expanded. RNA was extracted using RNeasy (QIAGEN), followed by synthesis of cDNA (Promega A3500) and amplification of VH and VL regions using leader family primers to VH2 (5′-ATGGACATACTTTGTTCCAC-3′) and VL8 (5′-ATGGCCTGGATGATGCTTCTCCTC-3′) and 3′ primers to γ (5′-GTCCTTGACCAGGCAGCCCAGGGCCGC-3′) and λ (5′-GCGTCAGGCACAGATAGCTGCTGGCCGC-3′) constant regions. The Vλ regions of hybridoma clone 2E12 and 2 nontumor-derived hybridomas were amplified with SMARTer RACE cDNA Amplification (Clonetech) in combination with the λ constant region primer. The same primers used for the PCR were used for sequencing.
Flow cytometry
HEp-2 cells were fixed and permeabilized (BD Cytofix/CytoPermBuffer), stained with recombinant tumor Ig at 150 μg/mL followed by 2-fold dilutions. Binding was detected with goat anti–human IgG-Alexa647 (Invitrogen), followed by analysis on a FACSCalibur 2-laser cytometer.
Detergent-adsorbed lysate of myoferlin-HA–transfected 293T cells was used in tumor staining and stimulation assays. Detergent-adsorbed lysate of untransfected 293T cells served as a negative control. Frozen biopsy cells were thawed and washed twice with PBS 0.5% BSA. For analysis of myoferlin binding to tumor, 100 μL of antigen prep was added to 5 × 105 biopsy cells and incubated for one hour at 4°C. Cells were washed twice and stained for surface markers CD20 (340954), CD3 (558117), IgM (561010), and λ (555796), all from BD Biosciences. Anti-HA (Abcam, ab72564) or anti–mouse IgG2a (Southern Biotechnology, 1080-09) was used to detect myoferlin-HA or myoferlin-mG2a, respectively. For stimulation assays, 5 × 105 biopsy cells in 100 μL RPMI with 10% FCS were rested at 37°C for 30 minutes. A total of 100 μL of antigen prep, or anti-IgG or anti-IgM (Southern Biotechnology, 2041-14 or 2022-14) at a final concentration of 10 μg/mL, was added to cells and incubated at 37°C for 45 minutes. Cells were then fixed with 1.6% paraformaldehyde for 5 minutes at room temperature. Cells were pelleted and permeabilized with 2 mL ice-cold methanol and incubated at −20°C for 10 minutes. Cells were washed twice then stained for CD3 and CD20 (BD Biosciences, 558021), and phosphorylated S6 ribosomal protein (Cell Signaling, 4854S). Cells were collected on an LSR II 3-laser cytometer (BD Biosciences), and data were analyzed with Cytobank software (cytobank.org).31
Results
Reactivity of recombinant FL Igs with human tissue antigens
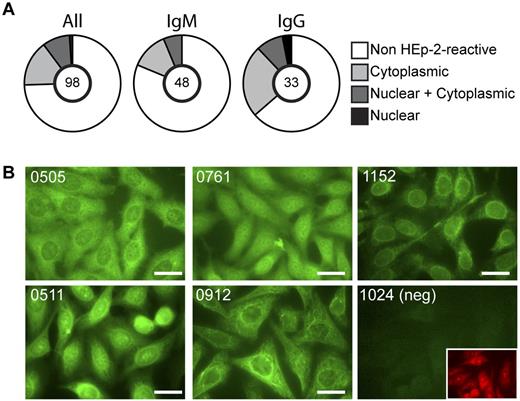
As expected, the tumor Igs were somatically mutated with an average of 34.7 and 20.8 mutations for heavy and light chain V genes, respectively (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Replacement mutations were more frequent in CDRs compared with FWRs (supplemental Figure 1B); however, improved methods for detecting antigen-driven selection did not identify positive selection for replacement mutations in the CDRs (supplemental Table 1). These results are consistent with previous analyses of tumor Igs.12,13,22 To test the recombinant tumor Igs for reactivity with a large collection of self-antigens, we used permeabilized human cells (HEp-2) as the target and performed a screen by IFA. HEp-2 IFA is a standard clinical assay for detecting self-reactive antibodies in the serum of autoimmune patients.32 All of the recombinant BCR proteins were expressed on a common IgG3 heavy chain backbone regardless of the original isotype of the tumor. The light chain constant regions, κ or lambda, matched those of the respective tumors. Therefore, all binding activity that differed between these recombinant tumor-derived BCRs could be attributed to their specific variable regions. The recombinant tumor Igs were reactive at a frequency of 25.5%, with 25 of 98 tested tumor Igs staining HEp-2 cells. When categorized based on the original isotype of the tumor, IgG tumors have a higher frequency of self-reactivity than IgM (Figure 1A). This frequency of HEp-2 reactivity is similar to that reported for normal antigen-experienced IgG and IgM B cells.33,34 There was great diversity in the observed staining patterns, indicating reactivity of different tumor BCRs with different cellular antigens. For example, the recombinant tumor Ig from patient 0912 stained filamentous structures, whereas the tumor Ig from patient 1152 gave a speckled staining pattern and appeared to bind in regions surrounding the nucleus and throughout the cytoplasm (Figure 1B).
Self-reactivity of recombinant FL Igs. Expressed Igs cloned from FL B cells were tested for self-reactivity by an indirect IFA on HEp-2 cells. (A) Summary of the frequency of self-reactive tumor Igs and the cellular localization of recognized antigens. The number of Igs tested is indicated in the center of the pie chart. (B) Examples of HEp-2 IFA staining patterns. Staining patterns of reactive Igs were confirmed in at least 2 independent experiments. Bars represent 25 μm.
Self-reactivity of recombinant FL Igs. Expressed Igs cloned from FL B cells were tested for self-reactivity by an indirect IFA on HEp-2 cells. (A) Summary of the frequency of self-reactive tumor Igs and the cellular localization of recognized antigens. The number of Igs tested is indicated in the center of the pie chart. (B) Examples of HEp-2 IFA staining patterns. Staining patterns of reactive Igs were confirmed in at least 2 independent experiments. Bars represent 25 μm.
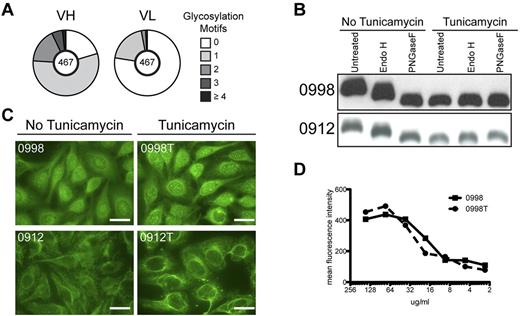
It has been reported that variable region SHM in FL introduces motifs available for N-glycosylation (Asn-X-Ser/Thr, where X is any amino acid except Pro, Asp, or Glu) and that the added glycans contain oligomannoses.35,36 We were able to confirm these observations. Of the tumor Igs studied here, 86% contain at least 1 N-linked glycosylation motif not encoded by the respective germline V gene. Acquisition of the motif was more prevalent in heavy chain variable regions compared with light chain variable regions with a frequency of 79.7% and 22.4%, respectively (Figure 2A). We studied several of these motif-containing Igs, and all were confirmed to contain high mannose sugars, as indicated by their sensitivity to Endo H, an enzyme that specifically cleaves oligomannose glycans. They also contained mature glycoforms as indicated by their sensitivity, and increased SDS-PAGE migration after digestion with PNGase F, which cleaves all N-linked glycans (Figure 2B). Oligomannose glycans were present in HEp-2 reactive and HEp-2 nonreactive tumor Igs, indicating that possession of oligomannose glycans does not dictate HEp-2 reactivity. However, for tumor Igs that contain oligomannose glycans, this sugar moiety might contribute to positive HEp-2 reactivity (ie, via lectin binding). To address this question, we generated tumor Igs that lacked N-linked glycans by growing the cells producing tumor Igs in the presence of tunicamycin, an inhibitor of N-linked glycosylation. Tumor Igs generated in these conditions were no longer sensitive to Endo H or PNGase F and had similar migration to PNGase F-treated Igs produced in the absence of tunicamycin (Figure 2B). Recombinant tumor Igs with and without N-linked glycans were then compared by HEp-2 IFA. We found that HEp-2 reactivity and staining patterns were unaffected by the removal of N-linked glycans (Figure 2C). Titration curves generated by intracellular flow cytometry were similar between tumor Ig with and without N-linked glycans (Figure 2D). These results demonstrate that the reactivity of these tumor-derived Ig proteins for antigens of Hep-2 cells was not dependent on carbohydrate moieties within the Igs.
HEp-2 reactivity is not dependent on variable region oligomannose glycans. (A) Number of N-glycosylation motifs in heavy (VH) and light (VL) chain variable regions, not encoded by the germline sequence. The number of sequences analyzed is indicated in the center of the pie chart. (B) Tumor Igs from patients 0998 and 0912 were produced in the absence or the presence of tunicamycin to generate Ig lacking N-linked glycans. Tumor Igs were then treated with Endo H or PNGase F to confirm the presence or absence of oligomannose glycans or N-linked glycans, respectively. Proteins were separated by SDS-PAGE and immunoblotted for human IgG. (C) HEp-2 IFA staining patterns of tumor Igs with and without (indicated by a “T”) N-linked glycans. Results are representative of 2 independent experiments. (D) Intracellular flow cytometric titration curve of HEp-2 cells stained with tumor Ig with and without (indicated by a “T”) N-linked glycans. Results are representative of 2 independent experiments.
HEp-2 reactivity is not dependent on variable region oligomannose glycans. (A) Number of N-glycosylation motifs in heavy (VH) and light (VL) chain variable regions, not encoded by the germline sequence. The number of sequences analyzed is indicated in the center of the pie chart. (B) Tumor Igs from patients 0998 and 0912 were produced in the absence or the presence of tunicamycin to generate Ig lacking N-linked glycans. Tumor Igs were then treated with Endo H or PNGase F to confirm the presence or absence of oligomannose glycans or N-linked glycans, respectively. Proteins were separated by SDS-PAGE and immunoblotted for human IgG. (C) HEp-2 IFA staining patterns of tumor Igs with and without (indicated by a “T”) N-linked glycans. Results are representative of 2 independent experiments. (D) Intracellular flow cytometric titration curve of HEp-2 cells stained with tumor Ig with and without (indicated by a “T”) N-linked glycans. Results are representative of 2 independent experiments.
Identification of myoferlin as a uniquely recognized self-antigen
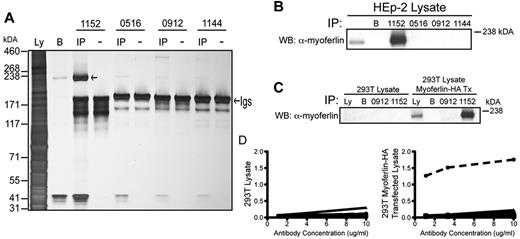
To identify HEp-2 antigens recognized by the self-reactive tumor Igs, all tumor Igs that were positive in the HEp-2 IFA were tested for their ability to immunoprecipitate protein antigens from HEp-2 cell lysate. The silver-stained protein band immunoprecipitated by the tumor Ig of patient 1152 was the most prominent and reproducible. For these reasons, we chose this tumor BCR and antigen pair for further study. The tumor Ig from patient 1152 immunoprecipitated a protein band just below the 238-kDA marker (Figure 3A). Using LC-MS/MS, we identified the immunoprecipitated protein as myoferlin, a protein known to associate with cell and nuclear membranes, which correlates with the HEp-2 staining pattern observed for patient 1152 (Figure 1B) and which is involved in membrane repair and VEGF signal transduction.28 The matched peptides covered 32% of the protein (supplemental Figure 2) and the calculated molecular weight of myoferlin is 236 kDA.
Identification of myoferlin as a uniquely recognized self-antigen. (A) Silver stain of a 3%-8% Tris-acetate gel of proteins immunoprecipitated (IP) from HEp-2 cell lysate by the indicated recombinant tumor Igs. (−) indicates lanes containing tumor Ig proteins only; IP, lanes containing the immunoprecipitated proteins; Ly, lysate; and B, lysate IP with protein G beads only. The left arrow indicates the 236-kDa protein immunoprecipitated by the tumor Ig of patient 1152; the right arrow points to the location of the tumor Igs. (B) Immunoblotting for myoferlin in immunoprecipitation samples from HEp-2 cell lysate. (C) Immunoblotting for myoferlin in immunoprecipitation samples from 293T cells transfected with recombinant myoferlin. (D) A total of 98 tumor Igs were tested for binding to recombinant myoferlin by ELISA. Myoferlin-HA was immobilized using anti-HA antibodies on lysates from untransfected (left panel) and transfected 293T cells (right panel). Shown is a representative graph of OD405-490 values for 14 different nonbinding patients' tumor Igs (solid lines) compared with tumor Ig for patient 1152 (dotted line). The ability of tumor Ig 1152 to bind myoferlin was confirmed in at least 2 independent experiments.
Identification of myoferlin as a uniquely recognized self-antigen. (A) Silver stain of a 3%-8% Tris-acetate gel of proteins immunoprecipitated (IP) from HEp-2 cell lysate by the indicated recombinant tumor Igs. (−) indicates lanes containing tumor Ig proteins only; IP, lanes containing the immunoprecipitated proteins; Ly, lysate; and B, lysate IP with protein G beads only. The left arrow indicates the 236-kDa protein immunoprecipitated by the tumor Ig of patient 1152; the right arrow points to the location of the tumor Igs. (B) Immunoblotting for myoferlin in immunoprecipitation samples from HEp-2 cell lysate. (C) Immunoblotting for myoferlin in immunoprecipitation samples from 293T cells transfected with recombinant myoferlin. (D) A total of 98 tumor Igs were tested for binding to recombinant myoferlin by ELISA. Myoferlin-HA was immobilized using anti-HA antibodies on lysates from untransfected (left panel) and transfected 293T cells (right panel). Shown is a representative graph of OD405-490 values for 14 different nonbinding patients' tumor Igs (solid lines) compared with tumor Ig for patient 1152 (dotted line). The ability of tumor Ig 1152 to bind myoferlin was confirmed in at least 2 independent experiments.
We confirmed the identity of myoferlin by performing immunoprecipitation from HEp-2 cell lysate, followed by immunoblotting with an antibody against myoferlin. (Figure 3B). To further validate the target of tumor Ig 1152, recombinant myoferlin containing an HA-tag was transfected into 293T cells, which do not express endogenous myoferlin. Immunoblotting for myoferlin confirmed its expression in transfected cells and immunoprecipitation of transfected cell lysate with tumor Ig 1152 showed enrichment of myoferlin, whereas immunoprecipitations with other tumor Igs did not (Figure 3C). To determine whether myoferlin could be a common self-antigen in FL, we used an ELISA where recombinant myoferlin was captured by antibodies against its HA tag. We tested all 98 tumor Igs; however, only the Ig from patient 1152 bound recombinant myoferlin (Figure 3D). These results indicate that myoferlin is a self-antigen, uniquely recognized by the BCR from the tumor of patient 1152. The apparent equilibrium affinity of tumor Ig 1152 for myoferlin was calculated as having KD of 10.3nM, as determined by biolayer interferometry.
Clonal analysis of self-reactivity and antigen binding
FL is characterized by ongoing SHM,5,6,10,11 and this phenomenon was confirmed in the tumor from patient 1152. The rearranged variable region was PCR amplified from DNA extracted from the tumor using 5′ FWRs1 and 3′ JH primers specific to the tumor variable region sequence. The PCR product was cloned and 52 molecular clones were sequenced. Among these 52 molecular clones, there were 19 unique sequences encompassing 30 mutations. There was a mixture of both silent and replacement mutations, which were distributed in both FWRs and CDRs. Importantly, no nonsense mutations were observed (supplemental Figure 3).
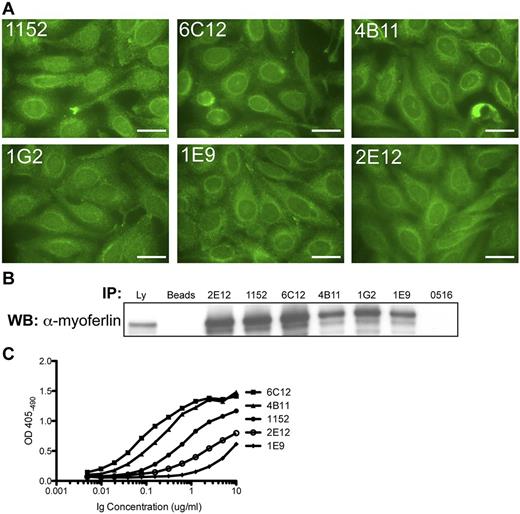
To determine whether self-reactivity or antigen binding was altered by ongoing SHM, we performed a rescue fusion on the tumor biopsy from patient 1152. This approach fuses the patient's cells with a heteromyeloma, which allows for the immortalization of individual tumor cells and the recovery of their Ig product, while halting SHM.37 A total of 5 tumor subclones were obtained, and they all contained tumor-derived Ig sequences but with different point mutations (supplemental Figure 4). Fusion clones 4B11 and 6C12 express heavy chain sequences that are identical to the 2 dominant molecular clones (supplemental Figure 3). Interestingly, clone 2E12 was strikingly different from the other recovered clones. Clone 2E12 shared with the other clones the tumor VH, DH, and JH genes and CDRs3 recombination joints, yet it had accumulated a great number of mutations that distinguished it from the other tumor clones. Clone 2E12 also expressed a different Igλ V gene. Based on the chromosomal location of Vλ and Jλ genes, the light chain genes of 2E12 were likely the genes expressed by the original premalignant B cell, whereas the light chain genes of the remaining tumor sublcones resulted from receptor editing in a precursor clone. Despite differences in the nucleotide and amino acid sequences of the recovered tumor subclones, all of them retained reactivity with human HEp-2 cells and had the same staining pattern (Figure 4A). All tumor subclone Igs were also able to immunoprecipitate recombinant myoferlin from the lysate of 293T cells transfected with the myoferlin-HA construct (Figure 4B). The tumor subclone Igs were also tested for binding to recombinant myoferlin by ELISA. Clones 4B11 and 6C12, which express heavy chain sequences that are identical to the 2 dominant molecular clones, exhibit the strongest binding (Figure 4C).
Igs of 1152 tumor subclones retain self-reactivity and antigen binding. (A) HEp-2 IFA staining pattern of tumor subclone Igs (2E12, 6C12, 4B11, 1G2, and 1E9) were obtained through rescue fusion of cells from the tumor biopsy of patient 1152; 1152 corresponds to the recombinant tumor Ig from patient 1152. Bars represent 25 μm. (B) Immunoblot for myoferlin in immunoprecipitations from lysate of 293T cells transfected with recombinant myoferlin-HA construct. Ly indicates lysate; B, lysate IP with protein G beads only; and 0516, an unrelated tumor Ig. (C) Tumor subclone Igs were tested for binding to recombinant myoferlin by ELISA. Recombinant Myoferlin-HA protein was immobilized using anti-HA antibodies on lysate from transfected 293T cells. Data shown are representative of at least 2 independent experiments.
Igs of 1152 tumor subclones retain self-reactivity and antigen binding. (A) HEp-2 IFA staining pattern of tumor subclone Igs (2E12, 6C12, 4B11, 1G2, and 1E9) were obtained through rescue fusion of cells from the tumor biopsy of patient 1152; 1152 corresponds to the recombinant tumor Ig from patient 1152. Bars represent 25 μm. (B) Immunoblot for myoferlin in immunoprecipitations from lysate of 293T cells transfected with recombinant myoferlin-HA construct. Ly indicates lysate; B, lysate IP with protein G beads only; and 0516, an unrelated tumor Ig. (C) Tumor subclone Igs were tested for binding to recombinant myoferlin by ELISA. Recombinant Myoferlin-HA protein was immobilized using anti-HA antibodies on lysate from transfected 293T cells. Data shown are representative of at least 2 independent experiments.
In addition to the tumor subclones, 2 clones of nontumor B cells were recovered from patient 1152. These nontumor B cell clones expressed different V genes and had different V/D/J or V/J joints for both IgH and Igλ, were unmutated, and were an IgM isotype, suggesting they were derived from normal mature naive B cells These normal B cell–derived Ig proteins exhibited completely different HEp-2 staining patterns and were unable to bind recombinant myoferlin (supplemental Figure 5).
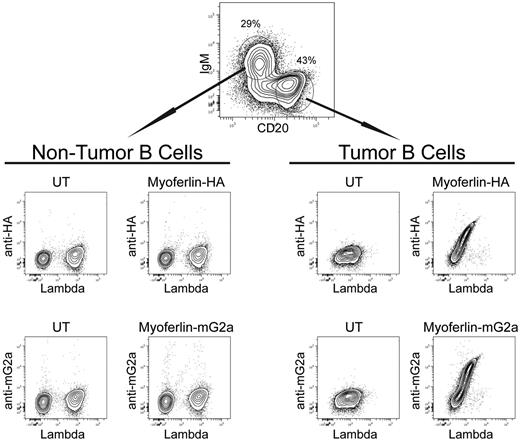
We next sought to assess the ability of the actual tumor cells from patient 1152 to bind myoferlin. We used 2 different recombinant myoferlin proteins (one containing an HA-tag, the other fused to the mouse IgG2a Fc) to stain a single-cell suspension of the biopsy from this patient. Tumor and nontumor B cells in the specimen were identified by a combination of CD20 and Ig isotype specific antibodies (supplemental Figure 6). Binding of the recombinant myoferlin antigen was detected with an antibody against the HA-tag or mouse IgG2a Fc. The tumor cells bound both recombinant forms of myoferlin in direct proportion to their level of BCR expression (Figure 5). The nontumor B cells showed no binding of myoferlin, once again providing a powerful internal control. These results indicate that despite the great intraclonal diversity generated by ongoing SHM, self-reactivity, and antigen binding were preserved and were a property specific for the Ig of the tumor cells.
Antigen binding by tumor cells of patient 1152 correlates positively with BCR expression. A single-cell suspension of the tumor biopsy was analyzed by flow cytometry to assess antigen binding by individual cells. Recombinant myoferlin with an HA tag or fused to mouse IgG2a FC was used to stain the cells. Antigen binding was detected with goat anti-HA or goat anti–mouse IgG2a. Lysate from untransfected 293T cells served as a negative control (UT). Cells are gated on CD3−CD20+ B cells; tumor B cells and nontumor B cells were identified with CD20hiIgM− and CD20intIgM+ gates, respectively.
Antigen binding by tumor cells of patient 1152 correlates positively with BCR expression. A single-cell suspension of the tumor biopsy was analyzed by flow cytometry to assess antigen binding by individual cells. Recombinant myoferlin with an HA tag or fused to mouse IgG2a FC was used to stain the cells. Antigen binding was detected with goat anti-HA or goat anti–mouse IgG2a. Lysate from untransfected 293T cells served as a negative control (UT). Cells are gated on CD3−CD20+ B cells; tumor B cells and nontumor B cells were identified with CD20hiIgM− and CD20intIgM+ gates, respectively.
Induction of BCR signaling of tumor cells by cognate antigen
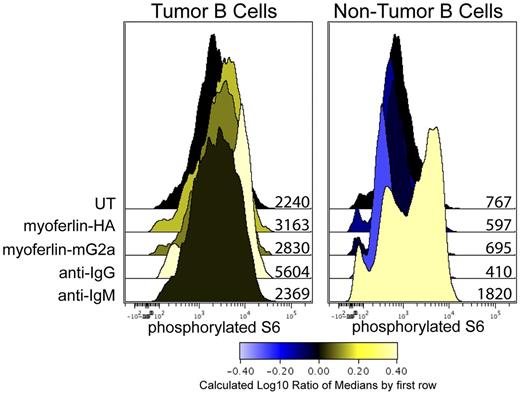
FL tumor cells are capable of signaling through their BCR after cross-linking of their BCR with polyclonal antibodies.38 Here we measured the levels of phosphorylated S6 ribosomal protein (S6), our most sensitive read-out of BCR-mediated signaling for this tumor. Cross-linking antibodies specific to the heavy chain isotype of the tumor and nontumor B cells, IgG and IgM, respectively, were used as a positive control for BCR-mediated S6 phosphorylation. When stimulated with recombinant forms of myoferlin, only the tumor cells showed an increase in phosphorylated S6 (Figure 6). The nontumor B cells only signaled in response to anti-IgM. Thus, cognate antigen was able to induce specific BCR signaling in the tumor cells.
Myoferlin stimulates phosphorylation of S6 ribosomal protein in tumor cells. A single-cell suspension of the tumor biopsy was stimulated with 100 μL of detergent-adsorbed lysate of either untransfected 293T cells (UT), or 293T cells transfected to express recombinant myoferlin containing an HA tag or myoferlin fused to mouse IgG2a Fc. A total of 10 μg/mL of goat anti-IgG and IgM was used as positive control for BCR signaling for tumor and nontumor B cells, respectively. Cells were stimulated for 45 minutes at 37°C. Cells were then fixed with 1.6% paraformaldehyde and permeabilized with methanol. Cells were stained for expression of CD3, CD20, and phosphorylated S6 ribosomal proteins. Tumor and nontumor B cells were identified with CD3−CD20hi and CD3−CD20int gates, respectively. Values adjacent to histograms indicate median fluorescent intensities.
Myoferlin stimulates phosphorylation of S6 ribosomal protein in tumor cells. A single-cell suspension of the tumor biopsy was stimulated with 100 μL of detergent-adsorbed lysate of either untransfected 293T cells (UT), or 293T cells transfected to express recombinant myoferlin containing an HA tag or myoferlin fused to mouse IgG2a Fc. A total of 10 μg/mL of goat anti-IgG and IgM was used as positive control for BCR signaling for tumor and nontumor B cells, respectively. Cells were stimulated for 45 minutes at 37°C. Cells were then fixed with 1.6% paraformaldehyde and permeabilized with methanol. Cells were stained for expression of CD3, CD20, and phosphorylated S6 ribosomal proteins. Tumor and nontumor B cells were identified with CD3−CD20hi and CD3−CD20int gates, respectively. Values adjacent to histograms indicate median fluorescent intensities.
Discussion
The hypothesis that antigenic stimulation can contribute to the development of B-cell malignancies was first proposed in 1959 in a discussion of proliferative similarities in autoimmunity and leukemia.39 Analysis of our large dataset of FL tumor BCR sequences revealed that replacement mutations accumulate in CDRs and are limited in FWRs, similar to previous reports,5 and consistent with normal antigen experienced B cells.33,34 These patterns of mutations, combined with the apparent selective pressure to preserve BCR expression, have been interpreted as evidence for antigen recognition in FL. However, newer methods that take into consideration the intrinsic mutational biases of the codons that comprise the CDRs and the FWRs have modified the view of how selective pressure by antigen can shape the mutational patterns of FL BCRs.12,13 These authors found evidence for negative selection against replacement mutations in FWRs but no evidence for positive selection for replacement mutations in CDRs. These results were interpreted to indicate the presence of a selective force to maintain the structural integrity of the BCR, perhaps to allow for tonic signaling, but not for specific antigen binding.
With the present study, we demonstrated that at least a subset of FL tumors are capable of recognizing self-antigens. The frequency of self-reactivity in FL is similar to that which has been observed for normal memory B cells,33 indicting that FL tumors arise from a B-cell population that has been selected by antigen. Our screen was designed to detect tumor BCRs that were reactive against self-antigens and just those that are expressed in HEp-2 cells. Thus, the tumor Igs classified as nonreactive may well be reactive against foreign or self-antigen targets not included in our screen. As we were able to document antigen reactivity in FL, the apparent lack of positive selection for replacement mutations in FL CDRs may not necessarily indicate a lack of antigen selection. Once an optimal strength of antigen interaction has been obtained, further replacement mutations could be disadvantageous as they may reduce the ability of the BCR to interact optimally with antigen. Indeed, this is hypothesized to be the case in some autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and myasthenia gravis.40,41
In this study, myoferlin was identified as an antigen that was uniquely recognized by one patient's tumor. Whereas myoferlin is not highly expressed in FL tumor cells,42 immunohistochemical staining of malignant lymphoma biopsies for myoferlin show scattered cell staining.43 Thus, myoferlin is present in the tumor microenvironment and can serve as a source of antigenic stimulation for the tumor cells.
We were unable to identify a shared antigen that was recognized by more than one patient's tumor, but we cannot exclude the possibility that one exists. In CLL, there is a restricted Ig repertoire and frequent use of BCRs with identical CDRs3 sequences.19-21 These stereotypic BCRs of CLL often react with the same auto-antigen, supporting a role for antigens in driving this malignancy. In FL tumors, the Ig V gene usage is representative of the general B-cell repertoire, and there are no reports of stereotypic BCRs. Thus, there is no molecular evidence for shared antigen recognition in FL. With the BCRs from FL, we observed staining patterns on HEp-2 cells that were quite diverse, attesting to the possibility that the antigens recognized by FL tumor BCR are stochastically determined, based on the Ig rearrangement that occurred around the time of the t(14:18) translocation, and unique to each patient.
By identifying an antigen recognized by the BCR of a patient's tumor, we were able to interrogate the significance of antigen recognition in the pathogenesis of FL. Receptor editing occurs early in B-cell development and alters the BCR specificity of autoreactive B cells. When occurring in mature B cells, receptor editing, or receptor revision, is thought to rescue B cells in which SHM has prevented expression of a functional BCR, or led to weak interaction with antigen.44 Receptor revision has been reported in B-cell lymphomas,45,46 and such an event occurred during the history of the malignant B-cell clone of patient 1152. Despite the extensive intraclonal diversity of the tumor from patient 1152, generated through ongoing SHM and receptor revision, tumor subclones remained self-reactive and bound myoferlin. Furthermore, tumor cells isolated from the patient's biopsy bound myoferlin, with the degree of binding correlating with BCR expression levels. These results strongly suggest that a selective pressure did exist to maintain antigen recognition by the BCR of this FL tumor. We were also able to demonstrate the ability of antigen recognition to induce BCR-mediated signaling in tumor cells, as assessed by phosphorylation of S6 ribosomal protein. Phosphorylation of ribosomal protein S6 correlates with increased translation of mRNA transcripts involved in cell cycle progression.47 Thus, as a result of antigen binding, the tumor may be receiving survival signal through its BCR, which could support the continued growth and expansion of the tumor.
Recent work by the Stevenson group has led to the hypothesis that FL tumor cells receive antigen-independent survival signals through their BCR. They found that SHM leads to the introduction of N-glycosylation motifs and that these motifs contain oligomannose glycans.35,36 We have confirmed these findings. These glycans may interact with mannose-binding lectins in the tumor microenvironment, which would allow for an antigen-independent route of BCR stimulation in FL.48 In our study, N-linked glycans did not dictate self-reactivity, and removal of glycans did not abrogate antigen binding. Thus, signaling via lectin or by antigen binding are both possible and tumor cells may receive survival signals through multiple modes of BCR stimulation.
As the expression of the BCR and it is ability to be engaged by antigens and/or lectins appears to be critical to the survival of FL tumor cells, interference with BCR signaling might be an effective therapeutic strategy. Indeed, clinical trials are now underway to test drugs that target components of the BCR signaling pathway.49,50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr William Sessa for the myoferlin-HA construct, Orr Sharpe and the Human Immune Monitoring Center at Stanford University for assisting with mass spectrometry analysis, Debra Czerwinski and Jonathan Irish for help with flow cytometric analyses, and Behnaz Taidi for assistance with the rescue fusion.
This work was supported by the National Institutes of Health (CA34233 and P30 DK56339), the Albert Yu and Mary Bechmann Foundation, and the Stanford Digestive Disease Center. M.J.S. was supported by Stanford National Institutes of Health/NCRR CTSA (UL1 RR025744) and the Lucile Packard Foundation for Children's Health (E.D.M.). K.L.S. is supported by the PhD Program in Immunology (training grant 5 T32 AI07290, “Molecular & Cellular Immunobiology”). R.L. is a clinical research professor of the American Cancer Society.
National Institutes of Health
Authorship
Contribution: K.L.S., M.J.S., J.S., and N.H.K. designed and performed experiments and analyzed the data; A.A.A. and C.L. assisted in sequence analysis; E.D.M. assisted in experimental approach and critically edited the manuscript; and K.L.S., S.L., and R.L. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Division of Oncology, Department of Medicine, Stanford University School of Medicine, Center for Clinical Science Research, 269 Campus Dr, Rm 1105, Stanford, CA 94305; e-mail: levy@stanford.edu.