Abstract
Abstract 1095
Refractory ITP can be very difficult to treat and may not respond to a single agent. Even the thrombopoeitic agents (TPO-A) and IVIG, which have response rates of 60–90%, are at the lower end of this range in the worst cases. Therefore combination therapy may be required. Previous studies using small numbers of patients have suggested CHOP (Figueroa,NEJM,1993); danazol and azathioprine[aza](Boruchov, Blood, 2007);3 low dose immunosuppressives (cyclosporine[CSA]-mycophenalate[MMF]-aza)(Arnold, Blood, 2010); and alemtuzumab-rituximab (Gómez-Almaguer, Blood, 2010). The latter 2 combinations tend to have overlapping mechanisms of effect; none of the 4 have been widely validated.
Patients with very difficult ITP were selected for combination treatment utilizing 3 different mechanisms: CSA, a T cell modulating agent; weekly injections of romiplostim [Nplate], a TPO-A; and as-needed IVIG. CSA was administered at sub-transplant doses aiming for 100–200 ng/ml to minimize toxicity. This retrospective study sought to analyze the treatment effect of the addition of CSA to IVIG and TPO-A in patients unresponsive to the latter agents. Patients were responders if they had higher platelet counts, required less frequent IVIG infusions, and/or used a lower dose or frequency of romiplostim while taking CSA.
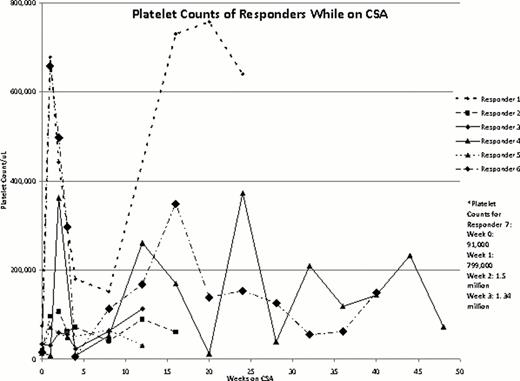
Nine patients with chronic ITP were treated with this CSA, romiplostim, and IVIG combination including 6 females and 7 patients who had undergone splenectomy. The average patient age was 41 yrs. Patients had received an average of 7.667 prior treatments for ITP and 8/9 patients were non-responders to TPO-A. Seven of 9 patients (4 females, 3 males) were responders. Two responding patients tapered to lower romiplostim doses, and 1 more completely discontinued romiplostim. Four required no IVIG after starting CSA and 1 patient required a single IVIG treatment 3 weeks into CSA treatment; the other 2 responding patients reduced their frequency and/or dose of IVIG therapy. All 7 responders achieved a platelet count ≥ 50 x103/uL and 6/7 ≥ 100 x103/uL. These 6 had > 50% of platelet counts ≥ 50 x103/uL. Four of 7 had > 50% of their counts ≥ 100 x103/uL. The 2 non-responders were both female and splenectomized. They continued to need rescue IVIG at similar intervals to those before CSA treatment; CSA was discontinued after 3–4 months.
Of the 9 patients on the study, 6 experienced headaches (4 responders) and 4 patients experienced abdominal discomfort. One responder discontinued CSA therapy after 12 weeks due to toxicity, which included headaches, increased irritability and worsening of preexisting molluscum contagiosum. There was not a clinically significant elevation of blood pressure in any responder; however, 1 non-responder with pre- existing treated hypertension had a clinically significant increase in blood pressure, which resolved upon discontinuation of the medication. None of the patients developed renal dysfunction or de novo infection while on combination therapy.
These results suggest that cyclosporine can be used as part of an effective combination with romiplostim and IVIG to manage patients with difficult ITP. The 3 agents were chosen to target different mechanisms of disease pathobiology: FcR “blockade” (IVIG), stimulation of platelet production (TPO-A) and inhibition of T cell activation (CSA). The efficacy of added CSA suggests that activated T cells contribute to refractoriness in difficult ITP. Future research would aim to identify patients in whom activated T cells play an important role and which T-cell agent, eg CSA, MMF or sirolimus, might be indicated in which patients.
Responder Data
| . | Sex . | Age (yrs) . | Number of Prior ITP Treatments . | Splenectomy (yes/no) . | Headache (yes/no) . | Abdominal Issues: Pain, Discomfort, Diarrhea (yes/no) . | Peripheral Neuropathy (yes/no) . | Significant Change In Blood Pressure (yes/no) . | IVIG while on CSA:1=none needed, 2=required 1 dose, 3= decreased frequency . | Decrease in romiplostim dose on CSA . | Baseline Plt Count (×103/uL) . | Peak Plt Count on CSA (×103/uL) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | F | 31 | 7 | Y | N | Y | N | x | 1 | No | 20 | 107 |
| Patient 2 | M | 3 | 6 | N | N | N | N | N | 1 | Yes | 14 | 373 |
| Patient 3 | M | 34 | 8 | Y | Y | N | Y | N | 2 | Yes | 5 | 730 |
| Patient 4 (discontinued CSA due to toxicity) | M | 6 | 7 | N | Y | Y | Y | N | 1 | No | 16 | 71 |
| Patient 5 | F | 77 | 7 | Y | N | N | N | x | 3 | No | 34 | 113 |
| Patient 6 | F | 66 | 7 | Y | Y | Y | N | N | 3 | No | 16 | 714 |
| Patient 7 | F | 24 | 8 | Y | Y | Y | N | N | 1 | Able to stop | 91 | 1.5 million |
| . | Sex . | Age (yrs) . | Number of Prior ITP Treatments . | Splenectomy (yes/no) . | Headache (yes/no) . | Abdominal Issues: Pain, Discomfort, Diarrhea (yes/no) . | Peripheral Neuropathy (yes/no) . | Significant Change In Blood Pressure (yes/no) . | IVIG while on CSA:1=none needed, 2=required 1 dose, 3= decreased frequency . | Decrease in romiplostim dose on CSA . | Baseline Plt Count (×103/uL) . | Peak Plt Count on CSA (×103/uL) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | F | 31 | 7 | Y | N | Y | N | x | 1 | No | 20 | 107 |
| Patient 2 | M | 3 | 6 | N | N | N | N | N | 1 | Yes | 14 | 373 |
| Patient 3 | M | 34 | 8 | Y | Y | N | Y | N | 2 | Yes | 5 | 730 |
| Patient 4 (discontinued CSA due to toxicity) | M | 6 | 7 | N | Y | Y | Y | N | 1 | No | 16 | 71 |
| Patient 5 | F | 77 | 7 | Y | N | N | N | x | 3 | No | 34 | 113 |
| Patient 6 | F | 66 | 7 | Y | Y | Y | N | N | 3 | No | 16 | 714 |
| Patient 7 | F | 24 | 8 | Y | Y | Y | N | N | 1 | Able to stop | 91 | 1.5 million |
”x” = insufficient data available.
Bussel:Amgen: Family owns Amgen stock Other, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; GlaxoSmithKline: Family owns GSK stock, Family owns GSK stock Other, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; IgG of America: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Portola: Consultancy. Off Label Use: The use of romiplostim in pediatric patients was examined in this study.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal