Abstract
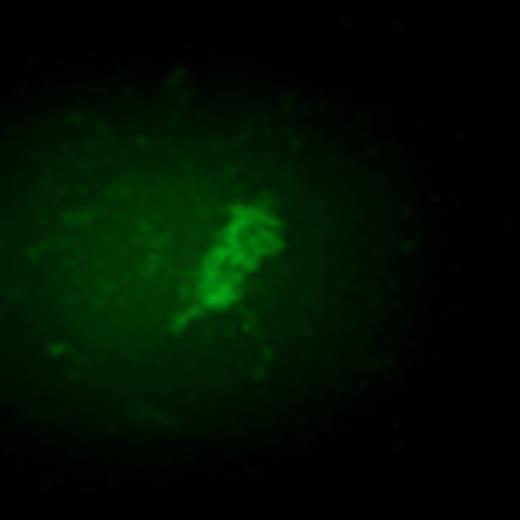
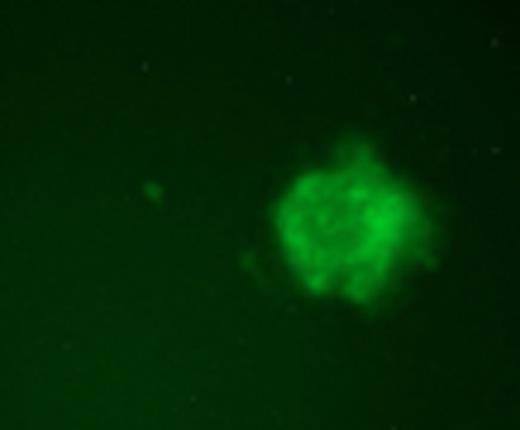
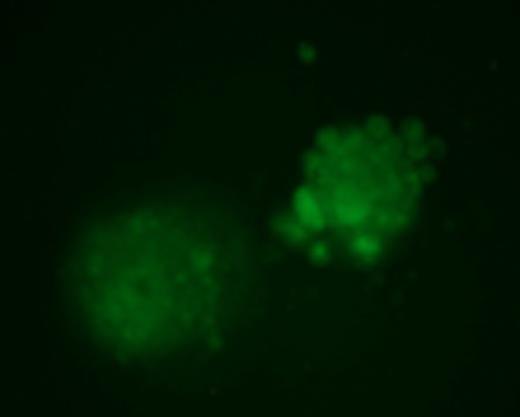
Infant acute leukemias account for ∼30% of all malignancy seen in childhood across the Western world. They are aggressive and characterized by rapid onset shortly after birth. The majority of these (∼80% ALL and ∼60% AML) have rearrangements involving the MLL gene. Although MLL fusion to more than 75 genes have been identified, AF9 is one of its most common translocation partners. MLL breakpoint sequences associated with infant acute leukemia are similar to those in secondary AML following exposure to the topoisomerase II (topoII) poison etoposide. This similarity led to the hypothesis that exposure during preganancy to biochemically similar compounds may promote infant acute leukemia. Some studies have shown an epidemiological link between bioflavonoid intake and increased incidence of MLL-rearranged infant leukemias. These bioflavonoids have also been shown to inhibit topoII in in vitro DNA cleavage assays and produce MLL rearrangements by inverse PCR in mice. Hundreds of unregulated nutritional supplements are widely available and perceived to prevent cardiovascular disease, inflammation, and cancer; however, their potential to promote leukemic translocations should be determined. Our goal was to create a model system that allows for rapid quantifiable screening of a large number of compounds to determine their potential to promote MLL-AF9 bcr translocations, and does not rely on inverse PCR that requires elimination of artifacts or less physiologically relevant internal deletions or intronic alterations from analysis. Thus, we developed reporter stem cell lines that contain two transgene constructs—(1) the MLL bcr fragment containing a genetically-engineered GFP 5' exon, and (2) the AF9 bcr containing a genetically-engineered GFP 3' exon. A translocation between the two bcrs reconstitutes the full-length GFP transcript resulting in quantifiable green fluorescence. Cells were treated with etoposide, quercetin, genestein, luteolin, dipyrone, or benzoquinone for 1 hour at 25mM – 200mM concentrations then allowed to repair and proliferate in culture. GFP+ fluorescent colonies as a result of MLL-AF9 translocations were readily scorable by 96 hours in a dose-dependent manner.
As previously demonstrated, totipotent stem cell viability was extremely impaired by multiple concentrations of etoposide, but the surviving fraction of hematopoietic stem cells that differentiated into multiple lineage sub-types exhibited a significant number of MLL-AF9 translocations (frequency of 1.5×10−4). Bioflavonoids genistein and quercetin (75mM) were also potent promoters of MLL-AF9 translocations in totipotent stem cells and hematopoietic stem cells at roughly similar frequencies as etoposide. By contrast, more differentiated hematopoietic progenitor cells had a significantly decreased (3-log) number of colonies with MLL-AF9 translocations following exposure to multiple compounds. This decrease is likely due to differences in DNA damage and repair responses or the potential of cells carrying translocations to proliferate in culture. The accuracy and significance of this model system is apparent from treatment of stem cells with benzoquinone that was not sufficient to produce MLL-AF9 translocations following exposure to concentrations up to 125mM (frequency < 0.1×10−6). Benzoquinone is a non-bioflavonoid thought to have a distinct mechanism of action and clinically associated with multiple leukemias. This system is a direct measure of the sensitivity of the MLL and AF9 bcrs to topoII poisons and bioflavonoids independent of their normal chromatin context and independent of formation of a leukemic fusion protein. In addition, the system allows for rapid and reproducible screening of hundreds of compounds that may have the potential to promote leukemogenic translocations in early stem cell and more differentiated cell subpopulations analogous to the events observed in infant acute leukemias.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal