Abstract
Abstract 1292
Notch signaling contributes to T cell leukemogenesis. However, we have found that activation of Notch signaling in human B-ALL promotes growth arrest and apoptosis. These contrasting effects of Notch in B versus T cell ALL, mirror effects seen in early lymphocyte development. As the Notch receptors are common between T and B cells, we hypothesized that these differences rely on the cell-type specific downstream mechanisms. We previously reported a critical role for Notch/HES1-mediated activation of Poly ADP-Ribose Polymerase 1 (PARP1) function in this B cell specific mechanism.
To explore the cell-type specific downstream mechanisms of Notch activation in B-ALL, we used cell fractionation, westerns and immunoprecipitation to identify cell cycle regulators which were altered by Notch activation via HES1 expression in human B-ALL lines.
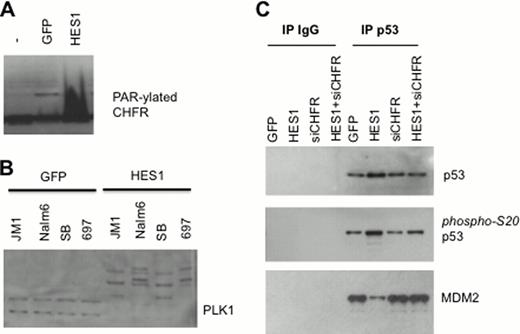
Notch activation in a panel of human B-ALL lines led to consistent growth arrest and apoptosis. Indeed, ligands, activated receptors and the Notch target gene HES1 all induced these leukemia lihibiting effects in B-ALL but not T-ALL lines. In this study we report a mechanism whereby HES1-mediated activation of PARP1 leads to PARylation of the E3 ligase Checkpoint with FHA and RING finger (CHFR) (Panel A) which results in targeting and ubiquitination of the cell cycle regulator Polo-Like Kinase 1 (PLK1) (Panel B). PLK1 is highly expressed in B vs. T-ALL and plays a critical role in B cell growth and survival. Following Notch activation, loss of ubiquitionated PLK1 through proteosomal degradation leads to cell cycle arrest through two mechanisms, namely cytoplasmic relocalization of cyclin B, disrupting the CDC2-cyclinB complex, as well as phosphorylation of p53 at S20, which leads to decreased weakened p53-MDM2 interaction and accumulation of p53 (Panel C). siRNA to CHFR reveal that this mechanism is dependent on CHFR (Panel C). Importantly this mechanism is not seen in T-ALL cells as the activation of PARP1 by HES1 does not occur in T-ALL cells.
Our findings reveal a novel molecular mechanism whereby Notch signaling induces disruption of the cell cycle in a cell type specific manner in B-ALL. Activation of PARP1, PARylation of CHFR, ubiquitination of PLK1 resulting in loss of nuclear cyclin B and accumulation of p53 demonstrates a series of events which can be initiated through activation of Notch in B-ALL. This mechanism reveals a potentially targetable approach to B-ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal