Abstract
Abstract 148
Successful therapeutic targeting of genetic lesions in cancer generally requires that such lesions be clonally dominant and uniformly represented. The commonest indolent non-Hodgkin's lymphoma (NHL) subtype, Follicular lymphoma (FL), is currently incurable with conventional regimes and is therefore an attractive candidate for targeted therapies. Our prior studies[1] and studies by others[2],[3] have shown that clinically significant variation in patient outcomes can be captured by differences in expression of CD20 both between tumor B-cells and between patients with NHL treated with or without rituximab. We hypothesized that a hierarchy of somatic mutations might exist in FL, and could impact the opportunity for future mutation-directed therapies in this disease.
Here, we examined clonal diversity in human Follicular Lymphoma (FL) using tumor subpopulations at diagnosis and at disease progression, through a genetic analysis of coding genomes and gene expression. We profiled whole-exomes and transcriptomes of 26 tumor-derived subpopulations sorted based on expression of CD20 in 10 lymphomas derived from 8 patients, including pairs at diagnosis and first disease relapse (mean exome coverage per patient >200x). We used phylogenies derived from ongoing somatic hypermutation of cloned immunoglobulin VH genes at diagnosis and relapse as a framework for comparing mutational hierarchies across the coding genome. We employed qPCR of invariant BCL2 and VDJ recombination to quantitate tumor purity in subpopulations.
We identified 882 somatic nucleotide variants (SNVs) encoding missense and nonsense mutations in 572 unique genes, with an average of 103 mutations/case, with 95% of mutations seen in only 1 case. Mutated genes were significantly enriched for those involved in chromosome and chromatin organization (FDR=4.07×10-4 and FDR=6.40×10-4, respectively). We identified mutations in many of the genes previously implicated in lymphomas, including MLL2 and CREBBP, and in several genes not previously implicated in lymphomas including IKZF2, CD40, CALR, NBPF14, ROS1 and ERBB2.
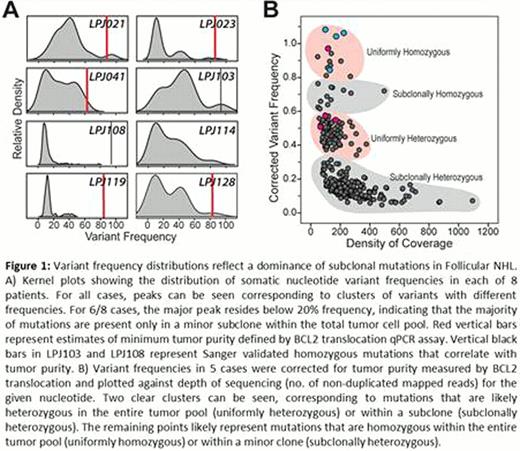
We observed 11–343 nonsynonymous coding mutations per tumor, with a striking tendency for most of these to be subclonal based on their low allelic variant frequencies (Figure 1 ). Many of these mutations were differentially distributed between tumor subpopulations which were evident both at diagnosis and at relapse. In addition, mutations in these populations were associated with distinct gene expression profiles. Surprisingly, subclonal distribution of mutations occurred in several genes which are known to be highly recurrently mutated in FL and thought to be drivers of pathogenesis. With rare exception, the frequency of mutation within a given gene across 375 patients with NHL studied by high throughput sequencing (FL=24, DLBCL=158, CLL=193) correlated poorly with clonal dominance within individual FL tumors in this study. By using immunoglobulin somatic mutations and BCL2 translocations as a frame of reference and by comparing diagnosis and relapse tumor pairs, we could distinguish early versus late somatic events during tumor evolution. We observed two contrasting patterns of clonal evolution, reflecting either maintenance or loss of most mutations at relapse. These two patterns were also mirrored by maintenance or loss of DNA copy number alterations in the respective cases.
These observations allow construction of genetic evolution models for FL. This framework provides important insight into lymphomagenesis and is a key step in prioritization of candidates for gene mutation-directed therapies.
No relevant conflicts of interest to declare.
References:
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal