Abstract
Abstract 1640
Two-year survival rates for adult BL remain <65% despite improvement in survival with intensive multi-agent chemotherapy. Furthermore, there remains a lack of prospective data examining the addition of rituximab (R) with CODOX-M/IVAC in BL and there is a paucity of available data regarding correlative plasma or cerebrospinal fluid (CSF) levels.
We enrolled 25 patients (pts) with classic BL onto a prospective phase II study (registered at clinicaltrials.gov [NCT00392990]). Pts were classified as low risk (LR) or high risk (HR); LR pts received 3 CODOX-M cycles, while HR pts had 4 alternating CODOX-M and IVAC cycles (Mead et al, Blood 2009). Liposomal doxorubicin (40 mg/m2) was used in lieu of doxorubicin, while intravenous R (500 mg/m2) was given days 0 + 8 of CODOX-M and days 0 + 6 of IVAC cycles. Correlative analyses of paired plasma and CSF rituximab levels were obtained from the first 10 pts for cycles 1 and 3 at 24 and 72 hours (hrs) after day 0 rituximab infusion. ELISA was used to detect rituximab in plasma and CSF. For assay validation, intra- and inter-day variability testing was performed through analysis of standard curve and three QC samples (high, medium, low concentrations) at least 3 times on the same and different days. A ten point standard curve was created by two-fold serial dilution of a 500ng/mL solution.
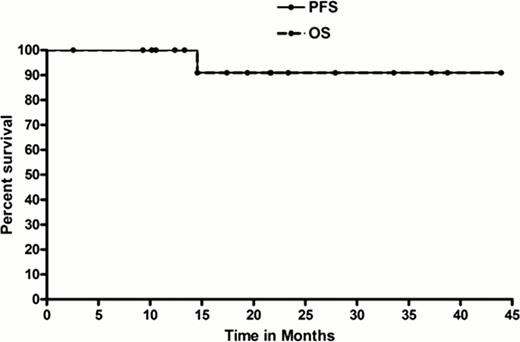
Median age was 44 years (yr) (23–70). There were 20 HR and 5 LR pts; 3 HR and 1 LR pt were HIV+. 15% of HR pts had + CNS disease. Additionally, 35% of HR pts had bulk >10 cm and 40% had bone marrow involvement. 24/25 pts were evaluable. Therapy was completed at a median of 13 weeks (11–20) for HR pts and 10 weeks for LR (9–12). Myelosuppression (62% grade 4 thrombocytopenia, 4% grade 4 anemia) and mucositis (33% grade 3, 13% grade 4) appeared comparable with prior CODOX-M/IVAC data. Other grade 3 toxicities were: infection (38%), neutropenic fever (29%), transaminitis (33%), diarrhea (8%), cardiac (8%), elevated creatinine (8%), seizure (4%) and nausea/vomiting (4%). Notably, no grade 3/4 neuropathy occurred. The objective response rate (ORR) after 2 cycles was 100% (67% complete remission). At a median follow-up of 34 months, 2-year PFS and OS rates for all pts were 86% and 86%, respectively (LR 2-yr PFS and OS: both 100%; and HR 2-yr PFS and OS: both 82%). Furthermore, as shown in Figure 1, the 2-yr PFS and OS for HR, HIV-negative pts were 91% and 91%, respectively (disease-specific survival 100%). Two pts died from progressive disease; both were HIV + HR pts. Regarding correlative studies, the median plasma rituximab levels at 24 and 72 hrs after day 0, cycle 1 rituximab infusion were 228,820ng/ml and 126,365ng/ml, respectively, while levels for cycle 3 (24 and 72 hrs after day 0 rituximab) were 246,200ng/ml and 199,700ng/ml, respectively. For paired CSF samples, rituximab levels were 104ng/ml and 227ng/ml, respectively, on cycle 1 and 260ng/ml and 248ng/ml, respectively, on cycle 3. This equates to plasma:CSF ratios for cycle 1 (at 24 and 72 hrs) of 0.04% and 0.18%, respectively, and for cycle 3 (24 and 72 hrs) of 0.10% and 0.20%, respectively. The median plasma and CSF rituximab levels for the 2 pts who relapsed (and died) were compared with pts without relapse (Table 1). Interestingly, cycle 1, 24-hr plasma R levels were significantly higher among pts without relapse compared with the two pts who relapsed/died (P=0.042); cycle 3, 24-hr serum R levels were of borderline significance (P=0.06). There were no differences identified regarding tumor burden of these 2 pts compared with other (non-relapsing) pts.
The integration of R into CODOX-M/IVAC for adult BL is feasible and associated with similar tolerability compared with prior reports. This regimen was associated with excellent survival rates, especially for HIV-negative BL. Further investigation of the predictive value of plasma R levels is warranted.
Rituximab Levels for BL Pts With and Without Disease Relapse
| Chemotherapy cycle/hours after R infusion . | Median serum R level (ng/ml) for pts without relapse . | Serum R level for 2 pts with relapse . | Median CSF R level (ng/ml) for pts without relapse . | CSF R levels for 2 pts with relapse . | ||
|---|---|---|---|---|---|---|
| Pt #1 . | Pt #2 . | Pt #1 . | Pt #2 . | |||
| C1/24h | 258,135 | 170,770 | 91,180 | 104 | 277 | 23 |
| C1/72h | 139,425 | 60,470 | 29,780 | 253 | 220 | 133 |
| C3/24h | 306,400 | 162,190 | 224,360 | 246 | 274 | N/A |
| C3/72h | 218,850 | 149,680 | 135,450 | 196 | 378 | 580 |
| Chemotherapy cycle/hours after R infusion . | Median serum R level (ng/ml) for pts without relapse . | Serum R level for 2 pts with relapse . | Median CSF R level (ng/ml) for pts without relapse . | CSF R levels for 2 pts with relapse . | ||
|---|---|---|---|---|---|---|
| Pt #1 . | Pt #2 . | Pt #1 . | Pt #2 . | |||
| C1/24h | 258,135 | 170,770 | 91,180 | 104 | 277 | 23 |
| C1/72h | 139,425 | 60,470 | 29,780 | 253 | 220 | 133 |
| C3/24h | 306,400 | 162,190 | 224,360 | 246 | 274 | N/A |
| C3/72h | 218,850 | 149,680 | 135,450 | 196 | 378 | 580 |
Abbreviations: C, cycle; h, hours; pt, patient; N/A, not available.
Off Label Use: Rituximab and liposomal doxorubicin for untreated Burkitt's lymphoma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal