Abstract
Abstract 1692
BCR-ABL1 kinase domain mutations are the most common known cause of resistance to tyrosine kinase inhibitors (TKIs) in CML. Some imatinib resistant mutations also confer resistance to second generation TKIs nilotinib and/or dasatinib. Therefore, it is recommended that mutation analysis be performed before changing therapy. However, BCR-ABL1 mutant clones are often de-selected upon TKI cessation or change of therapy, and may become undetectable (Hanfstein et al, Haematologica 2011). It is not known whether treatment discontinuation or long term alternative TKI therapy leads to eradication of these mutant clones. If mutant clones persist at sub-clonal levels they have the potential to be re-selected and expand clonally given favorable conditions, such as change to a TKI for which they confer resistance. We examined longitudinal data of patients with imatinib resistant mutations that became undetectable by direct sequencing to determine whether these “long dormant” mutations could reappear, and the circumstances related to reappearance.
All chronic phase patients who had been monitored at our institution since starting imatinib, and had mutations detectable by sequencing during imatinib therapy were analyzed; 49 patients, median follow up since starting imatinib was 4.3 years (range 0.6–11.6 years). Sensitive mutation analysis using mass spectrometry (detection limit 0.2% mutant) was performed at selected times when the mutations became undetectable by direct sequencing (detection limit 10–20%).
Of the 49 patients with mutations detected by sequencing during imatinib therapy, mutations became undetectable by sequencing in 21 patients (29 mutations), at a median of 2 months after changing therapy (range 1–20 months). This was associated with increased imatinib dose (3 mutations), stopping imatinib (2), hematopoietic cell transplant (6), chemotherapy (1), switching to nilotinib (3), or switching to dasatinib (14). All mutations that became undetectable by sequencing when the patient switched to nilotinib or dasatinib were those known to be sensitive to the inhibitor received (e.g. F359V in a patient treated with dasatinib).
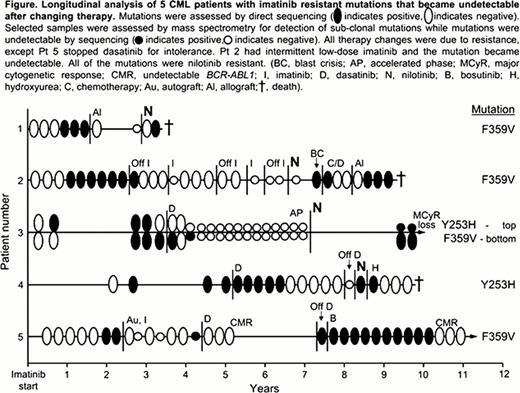
In 16 of the 21 patients whose mutations became undetectable by direct sequencing, the mutations have not been detected again with 0.1 to 6.9 years of follow up since the mutations were last detected (median 1.1 years). Of these 16 patients, 15 maintained a stable complete cytogenetic response and 1 lost a major cytogenetic response. In the other 5 patients, the same mutations as those originally detected (identical nucleotide exchange) became detectable by sequencing between 1.7 and 5.4 years after last detection (median 4.4 years), Figure. The original mutations in 4 of these patients confer resistance to nilotinib as well as imatinib (Y253H and F359V), and their reappearance was associated with initiation of nilotinib therapy, Figure. Three of these 4 patients died of their disease, and 1 lost a major cytogenetic response. Sensitive mutation analysis could detect the mutation in 1 of these patients during the time of “dormancy” and before nilotinib therapy. The 5th patient received an autologous hematopoietic cell transplant upon detection of F359V, and the mutation became undetectable by sequencing. The patient subsequently received dasatinib for 3 years and the mutation remained undetectable. Dasatinib therapy was stopped due to intolerance and F359V rapidly reappeared while the patient was off TKI therapy, having been undetectable for 4.8 years. Using sensitive mutation analysis, F359V could be detected at low levels after the transplant, suggesting that the mutant clone had not been eradicated.
The data suggest that some BCR-ABL1 mutations may persist at sub-clonal levels for many years after changing therapy. This could lead to clonal expansion under the selective pressure of a TKI for which the mutation confers insensitivity. Alternatively, the reappearance of the mutation could be a new occurrence of the same mutation. The study highlights the importance of knowing the mutation history of individual patients to enable informed therapy choices.
Yeung:Novartis Pharmaceuticals: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding. Hughes:Ariad: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Branford:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cepheid: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal