Abstract
Abstract 1736
Histone deacetylase inhibition (HDACi) has shown a potent inhibitory activity on the autonomous proliferation of hematopoietic cells of PV and ET patients carrying the JAK2V617F mutation (Leukemia. 2008;22(4):740–7). Hematological responses have been recorded during treatment with the HDACi givinostat in patients with PV and myelofibrosis (Blood. 2011; 118:a1748). Additionally, combinational use of givinostat and hydroxyurea (HU) have shown encouraging clinical response in PV (Br J Haematol. 2010;150:446–55). Recent laboratory studies of vorinstat-treated JAK2V617F knock-in mice have observed normalization of peripheral blood counts, markedly reduced splenomegaly and decreased mutant allele burden (Blood 2012; 119, 3779–89). Despite this evidence of hematologic response, to date no studies have evaluated specific changes in symptom burden among PV and ET patients treated with HDACi.
ET and PV patients from the UK were enrolled in a non-randomized, open-label phase II multicenter study of the HDACi vorinostat (Haematologica 2011;96(s2):a1023). Patients were asked to complete the 18 item Myeloproliferative Neoplasm Assessment Form (MPN-SAF) (Blood 2011;118:401–408), 9 item Brief Fatigue Inventory (BFI) (Cancer 1999;85:1186–1196) and 30 item EORTC QLQ-C30 (J Natl Cancer Inst 1993; 85(5):365-76) at enrollment, week 12, week 24, and week 36. From weeks 1 through 24, participants were given 400mg of vorinostat daily and were observed from weeks 24 to 36.
15 PV and 10 ET patients were enrolled in the study. Median age of participants was 65 and 48% of participants were female. JAK2 mutations were present in 5/10 (50%) of ET and 14/15 (93%) of PV patients.
Although most symptom severity changes did not reach statistical significance likely due to the small sample size, individual symptoms of headache (mean 1.6 baseline to 0.5 wk 36, mean maximum improvement 0.5 from baseline), dizziness (mean 1.6 baseline to 0.9 wk 36, mean maximum improvement 0.6 from baseline), sad mood (mean 2.6 baseline to 1.9 wk 36, mean max improvement 0.2 from baseline), and bone pain (mean 2.7 baseline to 0.8 wk 36, mean max improvement 0.7 from baseline) indicated consistent reductions in severity.
Non-sustained reductions in symptom incidence were seen with initiation of therapy for items of dizziness (50% baseline, 43% wk12, 55% wk 24, and 38% wk 36), numbness (65% baseline, 38% wk 12, 36% wk 24, 63% wk 36), sad mood (55% baseline, 47% wk 12, 40% wk 24, 50% wk 36), and itching (65% baseline, 50% wk 12, 40% wk 24, 75% wk 36).
Overall, MPN-SAF TSS did not vary significantly for the cohort over the course of the study (score change −.4 week 12, −0.5 week 24, −1.2 week 36, Figure 1). However, 9/17 participants who had a TSS of at least 6 at baseline had an overall improvement of at least 6 points (a moderate improvement) on at least one assessment after baseline. Of these, 5 of those 9 participants had an improvement of at least 10 points, which constitutes a large improvement in symptom burden based on a distributional definition of clinical significance using the standard deviation from a previous cohort.
This study represents the first analysis of symptom burden among PV and ET patients receiving vorinostat to date. In addition to previously identified changes in clinical and hematologic response, reductions in symptom burden including QOL and fatigue were observed in patients receiving vorinostat therapy which was most noticeable in patients with high baseline burden. The toxicity profile of vorinostat also suggests that better results might have observed if individualized assessment of clinicohematological responses including an integrated a marker for response into daily clinical practice and tailoring pharmacotherapy based on side effect profile (Leukemia. 2012;26(5):1148–9). Although further investigation among larger cohorts is needed, preliminary data is encouraging for successful therapeutic use of vorinostat in a clinical setting and with possible combinational therapy to reduce symptom burden among PV and ET patients.
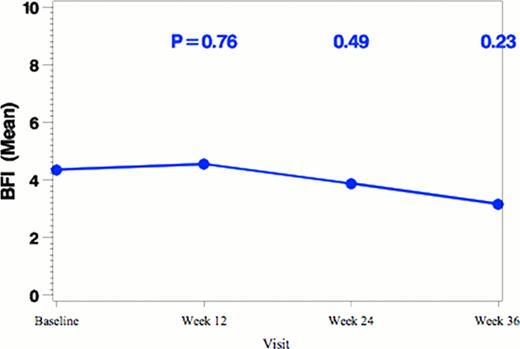
Changes of MPN-SAF TSS, BFI total score and overall QOL with vorinostat treatment (n=25).
Changes of MPN-SAF TSS, BFI total score and overall QOL with vorinostat treatment (n=25).
Harrison:Novartis: Honoraria, Research Funding, Speakers Bureau; YM Bioscience: Consultancy, Honoraria; Sanofi Aventis: Honoraria; Shire: Honoraria, Research Funding. Mesa:Incyte: Research Funding; Lilly: Research Funding; Sanofi: Research Funding; NS Pharma: Research Funding; YM Bioscience: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal