Abstract
Abstract 1741
Positron emission tomography (PET) generally employing fluorodeoxyglucose (FDG) combined with high-resolution structural imaging using computed tomography (CT) is regularly used in the diagnosis, staging and monitoring of treatment response in clinical oncology. 3′-18Fluoro-3′-deoxy-L-thymidine (18F-FLT) is a nucleoside analog that quickly accumulates in proliferating cells, more recently evaluated in various cancers including hematologic malignancies like acute leukemias or lymphomas as a PET radiotracer offering non invasive assessment of cell proliferation in vivo. Published results suggest that this technique could be useful to assess bone marrow (BM) activity and extramedullary hematopoiesis (EMH). However, to our knowledge, only 3 patients (pts) with myelofibrosis (MF) have been explored with 18F-FLT PET/CT (FLT PET). This pilot study aimed to establish proof-of-concept that FLT PET could be a new non invasive technique useful for MF management, in terms of diagnosis, staging and for monitoring response to therapy.
Pts were evaluated using 2 different techniques. First, conventional BM scintigraphy (BMS) was performed: on day 1, pts were injected with 99mTechnetium-nanocolloids and a planar image of the reticuloendothelial system was performed 30 min after injection; pts were then injected with 111Indium-Cl3 and planar imaging of the erythroid BM was performed after 48h. Secondly, FLT PET was performed 1 hour after injection of 18F-FLT (provided by AAA), and consisted in a whole-body acquisition. Images were interpreted in a blinded fashion independently by two nuclear physicians, qualitatively and according to a visual scale for both examinations. In addition, 18F-FLT uptake was quantified using standardized uptake value (SUV) in several sites of the skeleton, spleen and liver.
15 pts (9 men, 6 women, mean age: 62 years) were included between Apr 2011 and Jul 2012 (14 evaluable at time of abstract submission). 7 pts (47%) had primary (PMF), 4 post-polycythemia vera (PV), and 4 post-essential thrombocythemia (ET) MF, respectively (WHO criteria). All the pts had a BM biopsy with quantification of fibrosis. 11 pts (73%) had a JAK2V617F mutation, 1 a MPL515 mutation, and 3 had neither of these mutations. Therapies included hydroxyurea (n=1), androgens (n=1), interferon (n=4) and ruxolitinib (n=5); 4 pts had no specific therapy for MF.
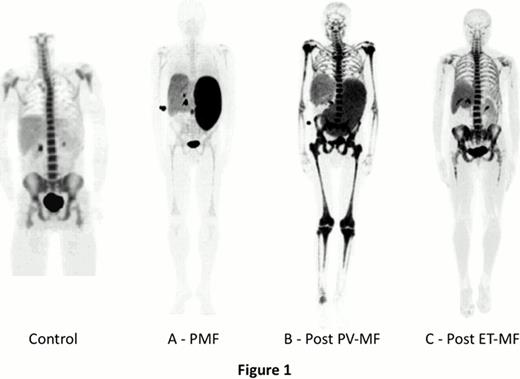
Three distinct patterns of FLT PET images were observed. 3 pts showed a marked reduced hematopoietic activity in the central compartment of the skeleton but a high uptake in spleen, suggesting the existence of myeloid metaplasia (Fig 1A). 8 pts had a rather normal pattern of BM activity in the central skeleton associated with marked expansion of BM activity to distal extremities and intense uptake of the tracer in the spleen (Fig 1B). 3 pts showed a relatively normal pattern of BM activity in the central skeleton, a mild expansion to distal extremities with no splenic abnormality (Fig 1C). FLT interpretation in myeloid malignancies is not standardized and we used comparisons with BMS to establish interpretation guidelines. Qualitative FLT PET results were equivalent to the 111In-Cl3 imaging in most cases, but in 2 pts FLT uptake was normal when BMS showed reduced 111In-Cl3 uptake. Compared to BMS, PET will also provide much more information including: (i) quantitative analyses of 18F-FLT uptake using SUV (preliminary results show that SUV ranges are [1.8 – 18.4] and [2.3 – 19.8] in BM and spleen, respectively); (ii) precise evaluation of malignant myelopoiesis in the different anatomical sites using coupled CT images. These analyses, and correlation with clinical and biological characteristics, BM histopathology and type of therapy received are ongoing.
FLT PET is a new, convenient non invasive technique for evaluation of malignant hematopoiesis in MF, including BM activity and EMH. Distinct patterns of FLT uptake may help in the diagnosis and staging of MF. In addition, ongoing correlation studies with histological BM fibrosis could provide evidence for a role of this non invasive technique in the assessment of the evolution of fibrosis over time without the need for sequential biopsies. A subsequent clinical trial will determine in a larger cohort of MF pts the usefulness of PET for evaluation of tumor response to therapy and prediction of early response using sequential evaluation of FLT uptake in BM and spleen.
Off Label Use: 3′-18Fluoro-3′-deoxy-L-thymidine (18F-FLT) is a nucleoside analog tested as a PET radiotracer in patients with myelofibrosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal