Abstract
Abstract 1866
This was a pilot study for a planned prospective trial of Bortezomib-Maintenance therapy to treat relapsed or refractory multiple myeloma (MM) patients. The purpose was to explore whether this maintenance therapy can lead to long-term progression free survival with good quality of life. We also analyzed and verified background factors that affected the continuity of maintenance therapy with Bortezomib using the data obtained.
Relapsed or refractory MM patients (N=38) who experienced at least one prior treatment and reached more than very good partial response (VGPR)/partial response (PR) after Bortezomib and Dexamethasone (BD) induction therapy between September 2008 and January 2012 were enrolled. All patients provided written, informed consent. The patients received Bortezomib once a month until progression disease (PD) as maintenance therapy (Clinical data cutoff date was May 2012).
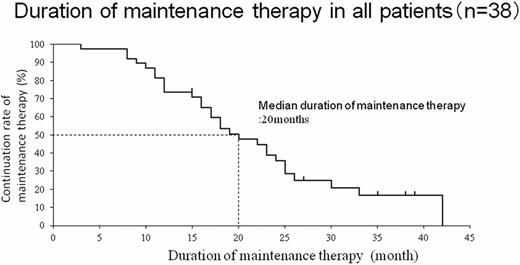
The average age of the patients was 64.8 years old (range, 29–86 years, 10 males, 28 females). The MM disease type classification was IgG-type in 23, IgA-type in 8, IgD-type in 1, and Bence Jones Protein(BJP) in 6. Therapy evaluation at the start of maintenance therapy was VGPR in 22 cases (58%) and PR in 16 cases (42%). The average period of continuous disease control was 20 months. Other than 2 cases of mild zoster and 4 cases of mild diarrhea, there were no G3/4 adverse events, including any peripheral neuropathy, which necessitated discontinuation of treatment during the maintenance therapy period. Statistical analysis according to patient age3 a 65 y/o and <65 y/o, ISS (I, II, III), the number of prior treatments (more than 1 or 2), period of induction therapy with Bortezomib, gender, presence or absence of complex karyotypic abnormalities, and time to progression (TTP) separated by a median of 24 months in disease duration was performed to determine whether any factor affected the continuity of maintenance therapy. A significant difference in the presence or absence of complex karyotypic abnormalities was observed. Slightly significant differences in ISS, the number of prior treatments, and the period of induction therapy with Bortezomib were also observed. The other factors did not affect the continuity of treatment.
The current results showed the median duration of maintenance therapy with Bortezomib was 20 months and the period of disease control including induction therapy was 26 months. We found that high TTP could be obtained by monthly maintenance therapy with Bortezomib, even when compared with the 6.2 months of TTP in the APEX global phase III trial that involved patients with relapsed and refractory MM treated with Bortezomib. The occurrence of peripheral neuropathy is thought to be associated with the dosing interval of Bortezomib, and the fact that monthly administration of Bortezomib decreases the risk of adverse events, including peripheral neuropathy, supports this. The present study confirmed the efficacy and safety of monthly administration of Bortezomib. Patients with complex karyotypic abnormalities may not be appropriate candidates for controlling the disease with monthly maintenance therapy. Thus, further studies on the dosing interval of Bortezomib during maintenance therapy are needed.
The results of the present study demonstrated the continuity and tolerability of monthly maintenance therapy with Bortezomib.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal