Abstract
Abstract 2046
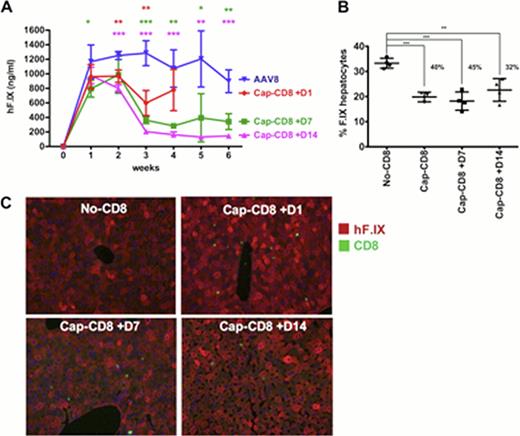
Long-term partial correction of severe hemophilia B following peripheral vein delivery of an AAV8-factor IX vector in human subjects has recently been reported. However, the two patients in the high-dose cohort experienced a rise in liver transaminases and drop in circulating F.IX levels that was halted with steroid treatment. In both the AAV8 and in an earlier AAV2-based trial, a dose of 2×1012 vg/kg seemed above a threshold for the activation of capsid specific memory CD8+ cytotoxic T lymphocytes (CTL). Therefore, reaching a target of > 5% sustained F.IX level (for a change to mild disease) is currently limited by activation of T cell immunity against capsid. New clinical trials are in the pipeline with AAV8 vectors expressing hyperactive F.IX variants that provide therapeutic F.IX expression at lower vector doses, with a goal of avoiding activation of CD8+ T cell memory response. Lack of a preclinical model to study CTL-mediated loss of AAV gene therapy has hampered efforts at clinical development. Neither mice nor non-human primates have recapitulated the human experience, making it difficult to evaluate, prior to clinical trial design, the effect of the serotype, vector dose, and other parameters of the protocol on targeting by capsid-specific T cells. To solve this problem, we have recently developed a murine model, in which male BALB/c RAG −/− mice receive hepatic AAV gene transfer followed by intravenous administration of in vitro expanded strain-matched capsid-specific CD8+ T cells (specific to an MHC I capsid epitope conserved between AAV2 and AAV8 serotypes shared between BALB/c mice and humans expressing the B*0702 molecule). In this model, AAV2-F.IX transduced mice showed a rise in liver enzymes, loss of circulating F.IX, and loss of F.IX expressing hepatocytes, following adoptive transfer of the CTL one day but not 7 or 14 days after gene transfer. CD8+ T cell infiltrates were observed 7 days following adoptive transfer and were absent at 28 days, suggesting a small window for optimal AAV2 capsid antigen presentation in the liver. Additionally, mice were protected from capsid specific CD8+ T cells when treated with the proteasome inhibitor bortezomib, which impairs the generation of peptide epitopes for MHC I antigen presentation. We next tested in our model AAV8 vectors, which in mice show superior tropism for liver. Published pre-clinical data by others suggested lack of capsid-specific CD8+ cell activation with this serotype. While this was not borne out in a clinical trial, the onset of T cell responses and of transaminitis in humans appeared to be delayed for AAV8 vector (8–9 weeks after gene transfer) compared to AAV2 (3–4 weeks). In comparison to AAV2, CD8+ T cell transfer in AAV8 injected mice had a milder impact on circulating F.IX levels (<50% loss of expression as opposed to 4-fold loss with AAV2), and CD8+ T cell infiltrates were largely absent at day 7. In two different experiments, 25–40% of F.IX expressing hepatocytes were lost compared to AAV8-F.IX transduced mice that received no or control CD8+ T cells. However, when the T cells were transferred 7 or 14 days after AAV8 administration, a more robust loss of systemic F.IX expression was observed (3- to 5-fold), with a 45% and 32% reduction in F.IX expressing hepatocytes, respectively (Fig 1 A-C). CD8+ T cell infiltrates were prevalent by day 42 in the livers of these animals. Together, these data suggest that optimal AAV8 capsid presentation in the murine liver occurs between days 28 and 42 following gene transfer. This delay in targeting of AAV8 transduced murine liver is consistent with the delay observed between the AAV2 and AAV8 F.IX clinical trials. This murine model should be useful to (1) evaluate novel AAV serotypes and capsid variants, (2) test the effect of the vector dose, (3) test the effect of pharmacological modulation on capsid presentation and targeting by capsid-specific CTL, and (4) provide guidance for the timing for immune suppression.
In vivo model for AAV8 capsid specific CD8 T cell response following AAV8 hF.IX liver gene transfer. (A) hF.IX levels (B) % hF.IX hepatocytes 42 days post vector (C) liver sections stained for hF.IX (red) and CD8 (green) 42 days post vector.
In vivo model for AAV8 capsid specific CD8 T cell response following AAV8 hF.IX liver gene transfer. (A) hF.IX levels (B) % hF.IX hepatocytes 42 days post vector (C) liver sections stained for hF.IX (red) and CD8 (green) 42 days post vector.
High:Amsterdam Molecular Therapeutics: ; Baxter Healthcare: Consultancy; Biogen Idec: Consultancy; bluebird bio, Inc.: Membership on an entity's Board of Directors or advisory committees; Genzyme, Inc.: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: ; Sangamo Biosciences: ; Shire Pharmaceuticals: Consultancy. Herzog:Genzyme Corp.: Royalties, AAV-FIX technology, Royalties, AAV-FIX technology Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal