Abstract
Abstract 3119
Hematopoietic stem cell transplants (HCT) for older patients with acute myelogenous leukemia (AML) have increased significantly over the past decade, associated with the use of reduced intensity conditioning (RIC) regimen. In the unrelated donor (URD) setting, anti-thymocyte globulin (ATG) has routinely been used to improve engraftment and decrease the incidence and severity of graft versus host disease (GVHD). We hypothesized that replacing ATG with low dose total body irradiation (TBI) during conditioning would provide sufficient immunosuppression to permit engraftment in older patients undergoing unrelated donor transplants for AML. Outcome data, including engraftment and GVHD rates were then compared to similar patients undergoing matched related donor (MRD) HCT for AML.
Between 2007–2012, 50 patients (55–70 years, median 61 years) with AML in CR1 (n=41) or >CR1 (n=9) were prospectively enrolled on an IRB-approved clinical trial using a RIC regimen. Donors were either MRD (n=23), or URD (n=27) if a suitable MRD was not available. HLA matched donors were available in 39 of the 50 patients, with single antigen mismatched donors used in 11 (41%) URD cases. All patients were conditioned with fludarabine (160 mg/m2) + busulfan (6.4 mg/m2) [FluBu2]; URD recipients additionally received 200cGy TBI on day -1 pre-transplant. GVHD prophylaxis was tacrolimus and mycophenolate. Patients exhibited high risk (n=21) or intermediate risk (n=29) disease at initial diagnosis, as defined by cytogenetic and molecular criteria. High risk (HR) patients included those with either a FLT3 mutation, monosomy 5 or 7, 5q-, 7q-, t(6:9) or complex cytogenetics (> 3 abnormalities). Patients with t(8:21), t(16:16) or inv 16 were excluded from analysis. Stem cell sources included peripheral stem cells (n=49) or marrow (n=1).
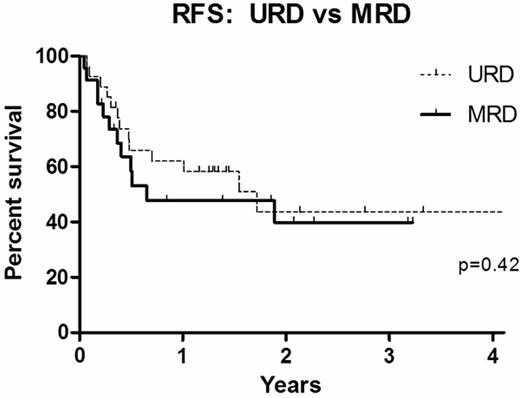
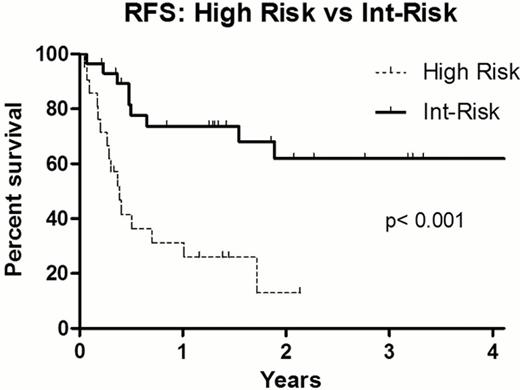
For the entire cohort, the 1y and 3y relapse free survival (RFS) was 56% (95% CI: 43–69) and 43% (95% CI: 28–58) respectively, with 1y and 3y overall survival (OS) rates 58% (95% CI:46–74) and 44% (95% CI: 27–62%). There was no significant difference in 1y RFS between URD and MRD recipients [62±9.5% vs 48±11%] or 3y RFS [44±11% vs 40±12%], p = 0.42 (Figure). There was no difference in outcome by age, with 3y RFS 42.5±11% for patients 55–59 yrs (n=20) and 40±12% for patients 60–70 yrs (n=30), p =0.54. The median donor age was 37 yrs (range 19–50) for URD and 58 yrs (range 42–70) for MRD recipients. The median CD34 cell dose infused was 5.0 × 10(6)/kg in both cohorts [range URD: 1.2 –8.3 × 10(6), MRD: 1.9 –9.8 × 10(6)]. There was no difference in time to neutrophil engraftment, with both cohorts engrafting a median 12 days post-transplant. Primary graft failure occurred in 1 (3.4%) URD recipient, who received an HLA mismatched graft. No cases of secondary graft failure occurred in either cohort. Day 100 CD33 chimerism was all donor in all URD cases, and in 75% of MRD recipients. Grade 2–4 acute GVHD developed in 44% URD and 30% MRD respectively, with grade 3–4 acute GVHD in 7% URD and 10% MRD. Patients with intermediate risk disease experienced 1y and 3y RFS of 74±8.5% and 3-year RFS 62±10.5%, respectively. By comparison, transplant outcomes were poor for patients with high risk disease, with 1y and 3y RFS only 31±10% and 13±10% (Figure).
Replacing ATG with low dose TBI in older AML patients undergoing RIC URD does not appear to compromise engraftment or increase GVHD rates. Survival is similar to that experienced by MRD recipients who are transplanted without either ATG or TBI. RFS for patients with high risk AML was poor, but patients with intermediate risk AML may benefit from this strategy when compared to historical results with non-transplant approaches for this patient population.
Talpaz:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; B.M.S.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Teva: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Couriel:Therakos: Speakers Bureau; Merck: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal