Abstract
Abstract 3258
Despite receiving occasional/no blood transfusions, non-transfusion-dependent thalassemia (NTDT) patients (eg β-thalassemia intermedia, mild/moderate HbE/β-thalassemia, α-thalassemia [HbH disease]) are at risk of iron overload, mainly due to increased intestinal iron absorption. The THALASSA 1-year randomized core study assessing deferasirox for investigational use in NTDT showed deferasirox superiority in reducing iron overload with a similar frequency of overall adverse events (AEs) compared with placebo (PBO). This 1-year extension evaluates continued efficacy/safety of deferasirox in patients receiving deferasirox in the core as well as patients receiving PBO who switched to deferasirox.
Study design/inclusion-exclusion criteria have been described (Taher et al. Blood 2012). In the prospective, open-label, 1-year extension, patients whose LIC decreased ≥30% vs baseline (BL) continued at the same dose received at core end of study (EOS). Patients with LIC 3–15 or >15 mg Fe/g dw received deferasirox 10 or 20 mg/kg/day in the extension, respectively. After 6 months extension, patients with LIC <3 mg Fe/g dw at month 18 interrupted treatment; patients with LIC >7 mg Fe/g dw at month 18 and with LIC reduction <15% compared with core EOS received dose escalation up to a max of 20 mg/kg/day provided no safety issues. If serum ferritin fell <100 ng/mL, drug was withheld. Primary efficacy endpoint was number of patients with LIC <5 mg Fe/g dw by extension end. “Deferasirox” patients refer to combined core + extension. Patients treated with PBO during the core who had the option to enter the extension and be treated with deferasirox are referred to as “PBO/DFX” patients.
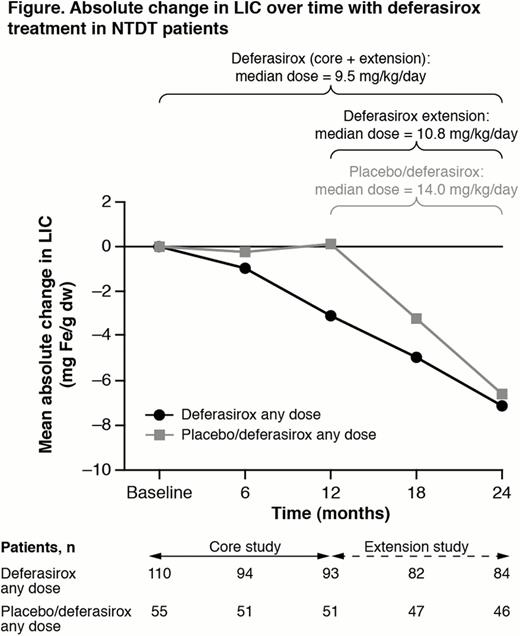
166 patients enrolled in the core study (deferasirox: 110; PBO: 56). 133/148 who completed the core entered the extension (deferasirox: 85; 48 PBO/DFX); 15 did not enrol in the extension (mainly due to extension amendment not approved when patients reached core EOS [n=8] and not interested in continuing [n=5]). 130 patients (84 [98.8%] deferasirox; 46 [95.8%] PBO/DFX) completed the extension; 1 patient discontinued in the deferasirox group (due to AE), 2 patients discontinued in the PBO/DFX group (due to AE [n=1], local admin problem [n=1]). Mean actual deferasirox dose was 9.8 ± 3.6 mg/kg/day (median 9.5) in core + extension; 12.6 ± 4.5 mg/kg/day (median 10.8) in the extension (n=82) and 13.7 ± 4.6 mg/kg/day (median 14.0) in patients who crossed over from PBO to deferasirox in the extension, notably higher than those continuing on deferasirox (Figure).
Mean absolute change in LIC from BL to month 24 was –7.14 mg Fe/g dw (Figure). By EOS, 43 (39.1%), 18 (16.4%) and 80 (72.7%) patients achieved a LIC<5, <3 and LIC decrease ≥3 mg Fe/g dw, respectively. In patients with BL LIC>7 mg Fe/g dw, 51/92 (55.4%) achieved LIC ≤7 at EOS. Median change in serum ferritin from BL to EOS was –450 ng/mL. Most common AEs regardless of study drug relationship: upper respiratory tract infection (20.9%), pyrexia (17.3%), diarrhea (13.6%), headache and nausea (both 12.7%), upper abdominal pain, anemia and gastroenteritis (11.8%); serious AEs (>2%): gastroenteritis (6.4%), pyrexia (3.6%), anemia (2.7%). 5 (4.5%) patients had serum creatinine increases >33% above BL and above ULN at ≥2 consecutive visits. One patient had ALT increase >5 × ULN and >2 × BL.
21 (37.5%), 6 (10.7%) and 36 (66.1%) patients achieved LIC<5, <3 and LIC decrease ≥3 mg Fe/g dw, respectively. In patients with BL LIC>7 mg Fe/g dw, 17/42 (40.5%) achieved LIC ≤7 at EOS. Patients who crossed over from PBO to deferasirox entered the extension with a higher extension BL LIC than those continuing on deferasirox. Most common AEs regardless of study drug relationship: pyrexia (26.8%), upper respiratory tract infection (25.0%), diarrhea, headache, nausea (21.4%), abdominal pain (16.1%), vomiting, cough (14.3%).
In iron-overloaded NTDT patients treated with deferasirox in the core study, LIC continued to decrease in the extension with 39.1% and 16.4% of patients reaching LIC <5 and <3 mg Fe/g dw, respectively, over 2 years. In patients originally randomized to PBO, whose LIC had increased by core EOS, LIC decreased with deferasirox in the extension. Data confirm deferasirox efficacy in reducing iron overload in NTDT, with a safety profile over 2 years consistent with that in the core study.
Taher:Novartis: Honoraria, Research Funding. Off Label Use: Exjade is indicated for the treatment of chronic iron overload due to frequent blood transfusions. This abstract describes off-label use of Exjade in patients with non-transfusion-dependent thalassemia syndromes with iron overload. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Viprakasit:GPO, Thailand: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Kattamis:Novartis: Honoraria, Research Funding, Speakers Bureau. Chuncharunee:Novartis: Research Funding. Sutcharitchan:Novartis: Research Funding. Siritanaratkul:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Galanello:Ferrokin: Research Funding; Apopharma: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Karakas:Novartis: Honoraria. Lawniczek:Novartis: Employment. Habr:Novartis: Employment. Ros:Novartis: Employment. Zhu:Novartis: Employment. Cappellini:Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal