Abstract
Abstract 3473
Diamond Blackfan Anaemia (DBA) is a rare bone marrow failure disorder characterised predominantly by severe erythroid (red blood cell) failure. While the causative mutation remains unknown in approximately 50% of DBA patients, a mutation resulting in haploinsufficient expression of one of a number of ribosomal proteins (RPS or RPL) has been identified in the remaining 50% of cases, leading to DBA being classified as a ‘ribosomopathy’(Dianzani & Loreni Haematologica. 2008). Activation of the p53 pathway in response to ribosomal disruptions and nucleolar stress has also been implicated in both animal and cellular models of DBA (Danilova et al. Blood 2008; McGowan et al. Nat. Genet. 2008; Fumagalli et al. Nat. Cell. Bio. 2009). Haploinsufficiency of ribosomal proteins appears to be the major cause of DBA although a recent study (Sankaran VG, et al. J. Clin. Invest. 2012) reported the discovery of mutations in the transcription factor GATA-1, in absence of any known ribosomal protein mutation. While GATA-1 mutations have only been detected in 2 DBA pedigrees to date, this exception to the ‘ribosomopathy’ rule may help identify the link between ribosomal protein disruption and the tissue-specificity of the erythroid defect.
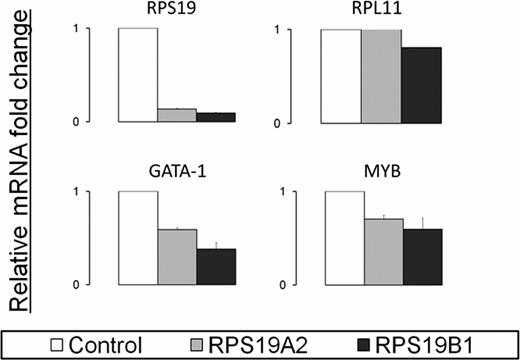
We have developed a Doxycycline-inducible shRNA RPS19 knockdown model of DBA in the human erythroleukaemic cell line TF1.8. Two independent shRNA triggers targeting RPS19 (the most common gene affected in DBA patients), and a vector-alone control were introduced into cells. RPS19 knockdown was validated by Q-RTPCR which showed a 5-fold decrease in RPS19 mRNA levels upon the addition of 0.25ug/ml Dox (Figure 1). Expression at the protein level was validated by western blotting. Other ribosomal proteins, for example RPL11, are unaffected.
The recent discovery of GATA-1 mutations in 2 unrelated DBA pedigrees led us to investigate whether the expression of GATA-1, or other key erythroid transcription factors, may be affected in our RPS19 DBA cell line model. Q-RTPCR results show decreased expression of two key transcription factors (GATA-1 and Myb) in cells with RPS19 knockdown compared to the controls (Figure 1). Furthermore, the expression of the GATA-1 target gene, FOG-1 is decreased compared to controls. MYBBP1A, a Myb target gene involved in ribosomal stress pathways (Yamauchi et al. Genes. Cells 2008) is increased, while another Myb target, GFI-1b is decreased in this model.
We have generated an inducible RPS19-knockdown model of DBA in TF1.8 cells, and have showed that several key transcription factors important in erythropoiesis (including GATA-1) display down-regulation associated with RPS19-knockdown. This suggests that changes to GATA-1 levels may be important not only in the rare cases of DBA where GATA-1 is mutated, but also in classical DBA associated with ribosomal protein haploinsufficiency. Based on the observation that GATA-1 associates with and inhibits p53 during normal erythropoiesis (Trainor et al. Blood 2009) we propose that the decreased GATA-1 expression may contribute to activation of the p53 pathway during impaired erythropoiesis in Diamond Blackfan Anaemia.
Further studies are being undertaken to test this model using assays for transcription factor activity, which will then be further characterised in a DBA model based on CD34+ cord blood cells infected with our validated RPS19 knockdown constructs.
Quantitative RT-PCR results showing the relative mRNA fold change of selected ribosomal proteins and key erythroid transcription factors in a Doxycycline-inducible RPS19 knockdown model of DBA in the human TF1.8 cell line. Data were normalised to β-actin mRNA expression. Charts reflect the combined data from three biological replicates (with the exception of RPL11 which is from a single biological replicate), with the relative fold change measured against vector-only control cells in the presence of Doxycycline.
Quantitative RT-PCR results showing the relative mRNA fold change of selected ribosomal proteins and key erythroid transcription factors in a Doxycycline-inducible RPS19 knockdown model of DBA in the human TF1.8 cell line. Data were normalised to β-actin mRNA expression. Charts reflect the combined data from three biological replicates (with the exception of RPL11 which is from a single biological replicate), with the relative fold change measured against vector-only control cells in the presence of Doxycycline.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal