Abstract
Abstract  365
365
Oral anticoagulation (OAC) therapy is the primary tool in reducing stroke risk in patients with non-valvular atrial fibrillation (NVAF), yet it is under-utilized. Patients that are non-persistent to therapy contribute to this underutilization. The recent introduction of dabigatran provides an alternative to warfarin for anticoagulation, but no studies to date have examined the relative patterns of persistence among patients starting therapy. The objective is to compare persistence rates between newly diagnosed AF patients new to OAC therapy on warfarin to dabigatran.
Claims data from the US Department of Defense was used to identify patients who received either dabigatran or warfarin between October 28, 2010 and June 30, 2012. Patient records were examined for a minimum of 12 months prior to their first prescription claim of dabigatran or warfarin to determine if they were naïve to treatment and if they were newly-diagnosed with atrial fibrillation (AF). If their first AF claim was within 3 months of their first dabigatran or warfarin prescription they are considered newly-diagnosed. Persistence was defined as time on therapy to discontinuation. Patients were identified as discontinued when the medication refill gap exceeded 30 days or if the patient switched therapies. Propensity score matching was used to compare cohorts. Kaplan-Meier curves were used to depict persistence over time. Cox proportional hazards model was used to determine the predictors of persistence, including stroke risk as estimated by CHADS2 score and bleeding risk as estimated by HEMORR2HAGES score.
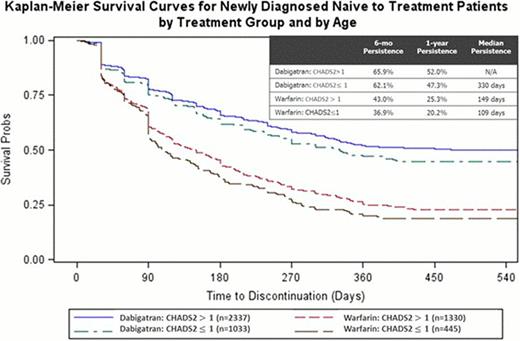
Fewer newly-diagnosed, treatment-naïve patients were started on warfarin (1,775) than dabigatran (3,370). Overall median persistence rates of patients on dabigatran or warfarin were 389 and 135 days, respectively. The persistence rates at sixmonths (64% vs. 50%) and 1 year (41% vs. 24%) were higher for dabigatran than for warfarin. Of those who discontinued, 9.4% switched from warfarin to dabigatran, and 5.9% switched to warfarin from dabigatran. Patients on dabigatran with a low risk of stroke (CHADS2 ≤1) or with a higher bleed risk (HEMORR2HAGES score greater than 3) had a higher likelihood of non-persistence (hazard ratio (HR), 1.1; 95% CI, 1.0 to 1.3; P=0.02 and HR, 1.2, 95% CI, 1.1 to 1.4; P= 0.004). Patients on warfarin with a low risk of stroke (CHADS2 ≤1) had 1.2 times higher likelihood of non-persistence (HR, 1.2, 95% CI, 1.0 to 1.3; P=0.04) (See Figure 1).
Patients who began dabigatran treatment were more persistent than patients who began warfarin treatment. Within each cohort, the patients with a low risk of stroke were more likely to discontinue therapy.
Francis:Trinity Partners, LLC: Boehringer Ingelheim sponsored the study Other, Consultancy. Siu:Boehringer Ingelheim Pharmaceuticals, Inc: Employment. Yu:Trinity Partners, LLC: Boehringer Ingelheim sponsored the study Other, Consultancy. Alvrtsyan:Trinity Partners, LLC: Boehringer Ingelheim sponsored the study Other, Consultancy. Rao:Trinity Partners, LLC: Boehringer Ingelheim sponsored the study Other, Consultancy. Walker:Boehringer Ingelheim Pharmaceutical Inc.: Employment. Sander:Boehringer Ingelheim Pharmaceutical Inc.: Employment. Zalesak:Trinity Partners, LLC: Boehringer Ingelheim sponsored the study Other, Consultancy. Miyasato:Trinity Partners, LLC: Boehringer Ingelheim sponsored the study Other, Consultancy. Sanchez:Trinity Partners, LLC: Boehringer Ingelheim sponsored the study Other, Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal