Abstract
Abstract 3692
Lenalidomide and ofatumumab have demonstrated clinical activity as single agents in a variety of types of non-Hodgkin lymphoma (NHL). This trial is a phase I/II trial combining these two agents for treatment of patients with relapsed and refractory B-cell NHL.
Patients with relapsed and refractory B-cell NHL of any histology were enrolled on a phase I/II trial combining lenalidomide and ofatumumab. Nine patients were on the phase I part of the trial and received a fixed dose of ofatumumab 1000 mg weekly × 8 doses along with lenalidomide 15 mg (N=3), or10 mg (N=6) for 21/28 days until the time of progression. The phase II portion of the study has 28 patients on the study with adequate follow-up at the time of analysis. The phase II doses were ofatumumab 1000 mg weekly × 8 along with lenalidomide 10 mg on 21/28 days. The lenalidomide was dose adjusted according to standard dose reduction criteria. All patients were on either a daily aspirin or other anticoagulation for thrombosis (DVT) prophylaxis.
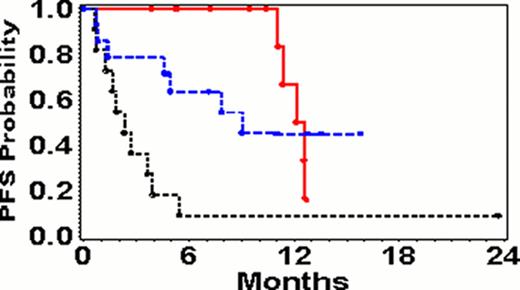
Thirty seven evaluable patients had adequate follow-up at the time of the analysis. The patients had a median age of 65 years (range 36–81), 76% were male, and 89% have an ECOG performance status of 0–1. The majority of patients had a relapsed indolent lymphoma with 12/37 (32%) follicular lymphoma (FL), 6/37 (16%) chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), 7/37 (19%) mantle cell lymphoma (MCL), one unclassifiable indolent lymphoma (3%), and 11/37 (30%) diffuse large B-cell lymphoma (DLBLC). The median duration of follow-up of surviving patients was 13 months (range 4–24). The complete response (CR) rate was 2/37 (5%) (one each FL and DLBCL) and the partial response (PR) rate was 13/37 (35%) for an overall response rate (ORR) of 15/37 (40%). The 1 year progression-free survival (PFS) was 41% (95% CI; 23–58) and the 1-year overall survival (OS) was 68% (95% CI; 49–82). In an analysis of response by patient variables, those significant included the patients with an FL histology (ORR 83%) vs. DLBCL (ORR 18%) or other(SLL, MCL, unclassifiable) (ORR 21%) (p= 0.001) and lactic dehydrogenase (LDH) normal (ORR 56%) vs. elevated (ORR 14%) (p= 0.01). In an analysis of variables for PFS, the variables with significance include diagnosis of FL (1-year PFS 67%) vs. DLBCL (9%) and SLL, MCL, unclassifiable (45%) (p=0.002), LDH normal (1-year PFS 55%) vs. elevated LDH (1-year PFS 19%), and number of prior chemotherapies 1–2 (1-year PFS 58%) vs. > 3 (1-year PFS 19%). Higher grade toxicities included grade 4 neutropenia in 9/37 (24%), one each of grade 4 bacteremia, one grade 4 DVT, stroke, and acute renal failure.
The combination of lenalidomide and ofatumumab was well tolerated by most patients. The patients with indolent NHL had a high response rate of 83% and a 1-year PFS of 67%.
Vose:Glaxo Smith Kline: Research Funding; Celgene: Research Funding. Off Label Use: Lenalidamide and Ofatumumab will be discussed for use in indolent and aggressive non-Hodgkin lymphoma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal