Abstract
Abstract 371
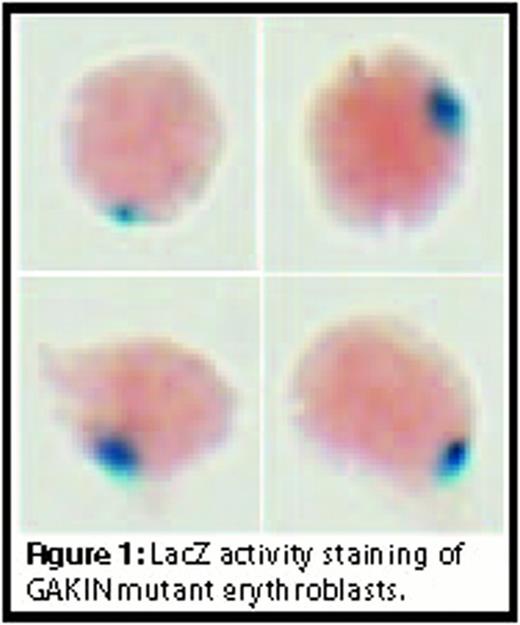
Terminal differentiation of mammalian erythroid precursors involves enucleation, a process required for the production of reticulocytes. The signaling pathways and the molecular components mediating the final enucleation step are not well understood, although the resemblance of enucleation to asymmetric cell division has been suggested. The enucleation of erythroblasts can be replicated in vitro; however, the enucleation efficiency is not optimal under in vitro culture conditions. A functional role of both microfilaments and microtubules has been suggested in the regulation of erythroblast enucleation. We hypothesized that molecular motors known to regulate asymmetric cell division might also play a functional role in erythroblast enucleation. GAKIN (also called KIF13B) is a kinesin-3 motor implicated in the regulation of cell polarity pathways. Its Drosophila homologue Khc-73 regulates polarity formation during the asymmetric cell division of neuroblasts. We generated a mutant mouse line of GAKIN and examined the efficiency of erythroblast enucleation using the fetal liver in vitro erythroid precursor culture system originally developed by the Lodish group. The GAKIN mutant mouse line was produced from the embryonic stem (ES) cells containing a genomic insertion of the beta-galactosidase-neomycin gene. The beta-geo insertion was mapped within intron 38 of the GAKIN gene, thus removing the CAP-Gly domain located at the C-terminus of GAKIN. This allowed us to visualize specific tissue expression of GAKIN, in addition to the subcellular localization of a GAKIN-lacZ fusion protein by lacZ activity staining. The lacZ activity revealed that the GAKIN-lacZ fusion protein is expressed in bone marrow macrophages and erythroblasts, while also highly concentrated at contact sites between macrophages and erythroblasts. GAKIN mutant mice are viable and fertile, and complete blood analysis did not reveal any discernible phenotype. To test for the enucleation efficiency, erythroid precursors were isolated from fetal liver stage at day 14.5 embryos and enucleation was quantified by flow cytometry after 2 days of in vitro culture. The GAKIN-lacZ fusion protein appeared as a single dot representing the microtubule organizing center (MTOC) in the erythroid precursors isolated from fetal liver (Fig. 1). However, GAKIN mutant erythroblasts enucleated at the same efficiency as wild type erythroblasts under steady state conditions in vitro. Since the mammalian genome contains a close homologue of GAKIN/KIF13B, termed KIF13A, a possibility exists that KIF13A functionally compensates for GAKIN mutation in critical pathways. To address this issue, we generated KIF13A null mice using a similar gene disruption strategy. Again, the KIF13A mutant erythroblasts did not show any measurable change in enucleation efficiency under similar conditions. However, fetal liver erythroblasts isolated from GAKIN and KIF13A double mutant mice exhibited a significant enhancement of enucleation efficiency. This finding suggests that together, GAKIN and KIF13A negatively regulate the erythroblast enucleation by modulating the microtubule-based cytoskeleton. We propose that efficient erythroid enucleation in vivo involves signaling mechanisms inhibiting both GAKIN and KIF13A motors. Since erythrocytes from adult double mutant mice appear to be hematologically normal, our findings raise the possibility of enhancing the in vitro production of functional erythrocytes by inhibiting GAKIN and KIF13A activity in CD34 positive stem cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal