Abstract
Abstract 3713
The complementarity determining region, or idiotype, of the surface immunoglobulin receptor is a tumor-specific marker on B-cell lymphomas that is unique to each patient. Antibodies against idiotype can induce complete regression of lymphoma in patients, but since this therapeutic approach requires the generation of a custom monoclonal antibody for each patient, it has not been practical.
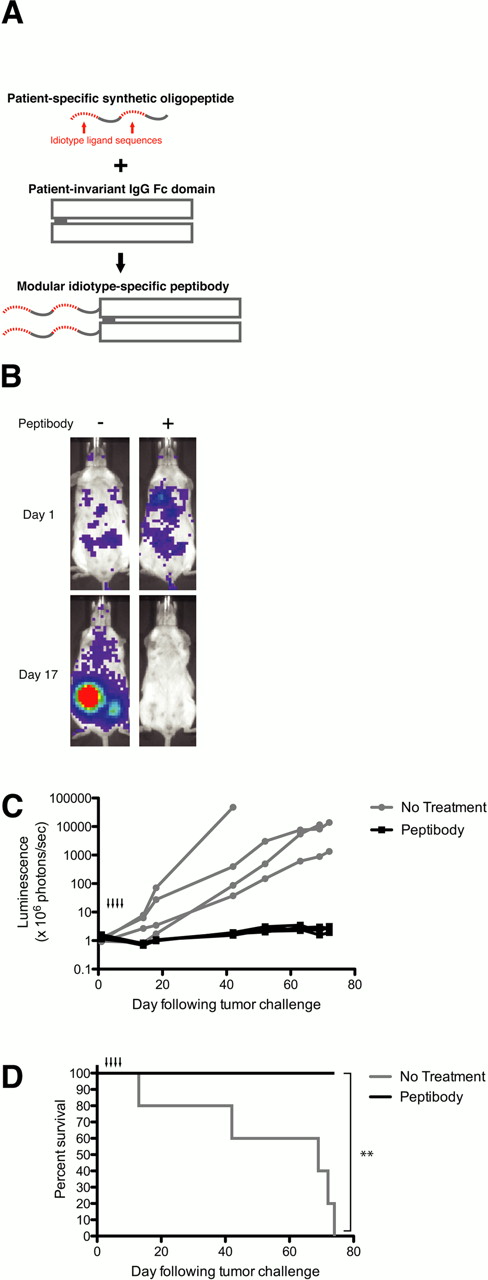
Here we describe a method for targeting the idiotype on the surface of a B-cell lymphoma by using synthetic idiotype-ligands covalently linked to a recombinant IgG Fc domain (Figure 1A). These peptide idiotype-ligands can be identified through oligopeptide library screens and produced inexpensively by automated solid-phase synthesis. Linkage of idiotype-ligands to the Fc domain serves two purposes: to enhance their pharmacokinetics and to augment their anti-tumor effect by activating immune effector functions. Since each patient-specific peptide can be chemically linked to a common IgG Fc domain, this modular construct design yields a patient-specific therapeutic that does not require the production of a custom biologic macromolecule for each patient.
Idiotype peptide-ligands were produced by solid-phase synthesis and covalently linked to the amino-terminus of a recombinant mouse IgG2a Fc domain by native chemical ligation, a method for site-selective polypeptide ligation. The resulting peptibody demonstrated targeted killing of a human lymphoma cell line in vitro by crosslinking surface immunoglobulin and triggering activation-induced death. Additionally, the peptibody triggered complement-mediated cytotoxicity of opsonized lymphoma cells in vitro and activated natural killer cells co-cultured with opsonized lymphoma cells. The peptibody exhibited a favorable pharmacokinetic profile and peptibody treatment was sufficient to clear tumor in SCID mice challenged intravenously with a luciferase-labeled human lymphoma cell line (p = 0.0018; Figure 1B-D).
Idiotype-specific peptibodies demonstrate multimodal activity against lymphoma cells in vitro and clear human lymphoma in a disseminated xenograft model. The modular design of this therapeutic may enable a personalized and targeted therapy that is feasible to produce for patients with B-cell lymphoma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal